Alto Neuroscience IPO investment analysis
January 16, 2024
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or an ongoing business relationship with the company.
Alto Neuroscience is a clinical-stage biopharmaceutical company developing precision medicines for psychiatry. Using a Precision Psychiatry Platform, Alto Neuroscience employs neurocognitive assessments, electroencephalography (EEG), and wearable devices to identify brain-based biomarkers and develop tailored treatments for specific patient populations.
The company's pipeline includes five clinical-stage assets targeting major depressive disorder (MDD) and schizophrenia. These programs, backed by Phase 1 tolerability data, are undergoing or approaching Phase 2 trials. Notably, ALTO-100 and ALTO-300 are entering Phase 2b trials with topline data reports expected in late 2024 and early 2025, focusing on subsets of MDD patients characterized by specific cognitive and EEG biomarkers. Initiations of Phase 2 proof-of-concept trials for ALTO-101 (for cognitive impairment associated with schizophrenia) and ALTO-203 (for MDD patients with high levels of anhedonia) are planned for the first half of 2024, with results anticipated in 2025.
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| ALTO-100 | Small molecule | BDNF Activator | Major Depressive Disorder with impaired cognition | |||||||
| ALTO-100 | Small molecule | BDNF Activator | Post-traumatic stress disorder with impaired cognition | |||||||
| ALTO-300 | Small molecule | MT1/MT2 agonist and 5-HT2C antagonist MT1/MT2 agonist and 5-HT2C antagonist | Major Depressive Disorder | |||||||
| ALTO-101 | Small molecule | PDE4 Inhibitor | Cognitive impairment associated with schizophrenia | |||||||
| ALTO-203 | Small molecule | H3 receptor Inverse agonist | Major Depressive Disorder with Anhedonia | |||||||
| ALTO-202 | Small molecule | GluN2B-NMDA receptor Antagonist | Major Depressive Disorder |
Risks and highlights
Biomarker-defined patient populations improve probability of success
Encouraging early clinical data
Multiple upcoming near-term milestones
Targeting large patient populations
Psychiatric disorders are heterogenous and therapies may not be effective for all patients
Early clinical data is from small-scale, observational clinical studies and larger studies may not replicate positive early data
Clinical development in MDD and other psychiatric indications is high-risk
Valuation
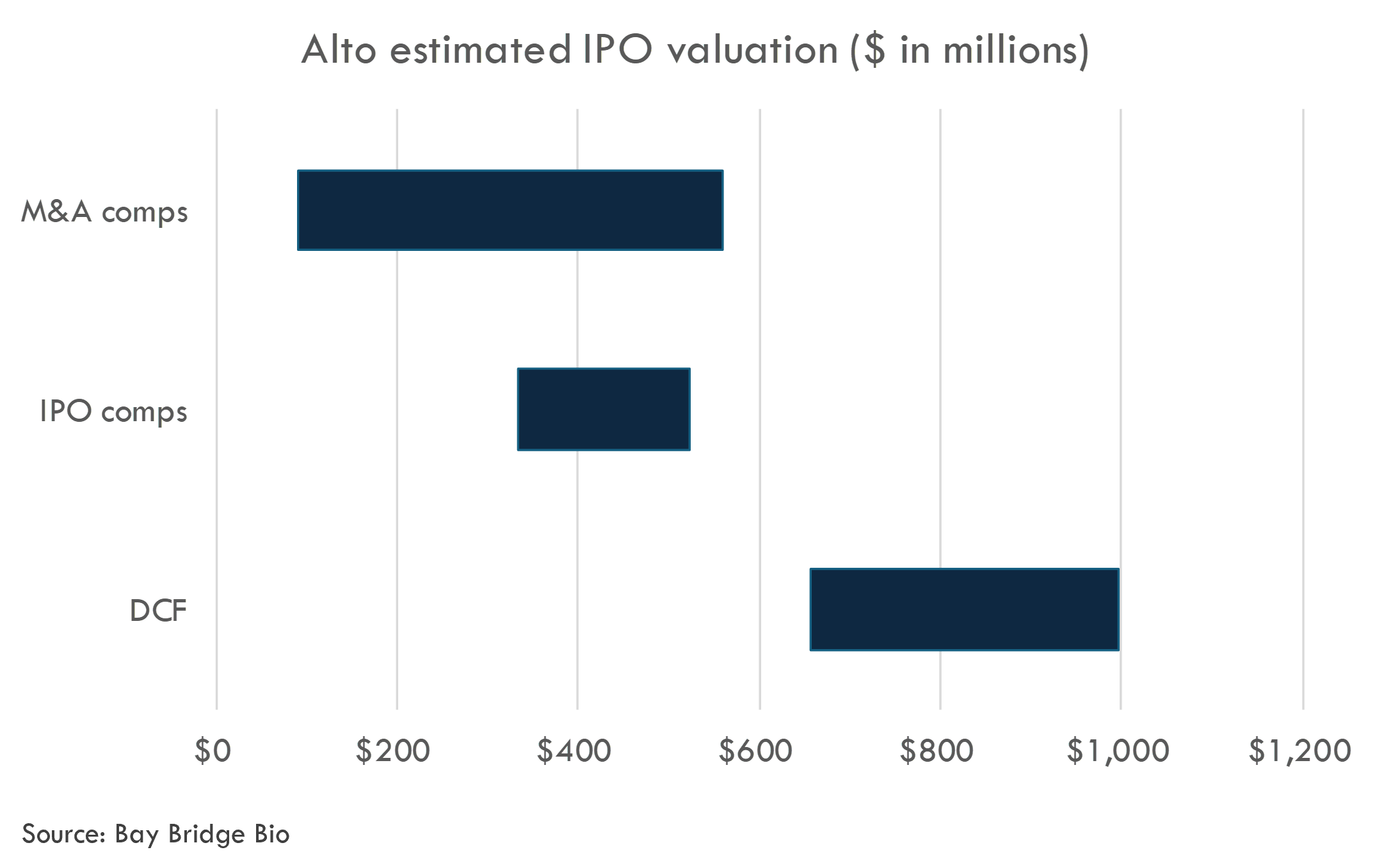
We estimate Alto's last private round valued the company at $207M. We estimated an IPO pricing range of $335-525 million.
ALTO-100
Therapeutic rationale
The therapeutic rationale for a BDNF activator like ALTO-100 in Major Depressive Disorder (MDD) with impaired cognition and Post-traumatic stress disorder (PTSD) with impaired cognition centers around the critical role that BDNF plays in neuroplasticity and neurogenesis.
In MDD and PTSD patients with impaired cognition, there is evidence of reduced neuroplasticity, particularly in the hippocampus—a region of the brain essential for learning, memory, and emotional regulation. A smaller hippocampal volume and decreased BDNF levels are associated with poor cognition and greater treatment resistance in these patients. The lack of neuroplasticity can contribute to the rigidity of negative thought and behavior patterns seen in depression and PTSD, which can exacerbate the conditions.
BDNF is a neurotrophic factor that is known to influence the survival, growth, and differentiation of neurons. It is crucial in synaptic plasticity, where it enhances the formation and strength of synaptic connections between neurons, and in neurogenesis, facilitating the birth of new neurons within the adult brain. The "neurotrophin hypothesis of depression" proposes that a deficiency in BDNF leads to the aforementioned neuroplasticity impairments, contributing to the symptoms of MDD and possibly PTSD with impaired cognition.
ALTO-100 has been observed to enhance neuroplasticity at the synaptic and cellular levels and to promote neurogenesis in preclinical models. In these models, the drug has been shown to increase hippocampal synaptic plasticity, which, over prolonged exposure, leads to improvements in synaptogenesis (formation of new synapses) and neurogenesis, as well as an increase in hippocampal volume. These neuroplastic changes could, therefore, counteract the negative impact of impaired cognition seen in MDD and PTSD.
The Phase 2a clinical trial results suggest that patients with MDD characterized by objectively measured cognitive impairments responded better to ALTO-100 than those without such impairments. This response was measured by an improvement in depressive symptoms, indicating that ALTO-100’s pro-neurogenic and neuroplastic mechanisms of action might be particularly beneficial for this subset of patients.
Given this background, the therapeutic rationale for ALTO-100 is founded on the idea that restoring or enhancing BDNF signaling and thereby reversing the deficits in neuroplasticity can significantly improve cognitive function and reduce depressive symptoms in patients with MDD and potentially in patients with PTSD who exhibit impaired cognition. If approved, ALTO-100 might serve as a first-in-class treatment for this indication, offering a novel approach to targeting the underlying pathophysiology of these disorders.
The science surrounding the role of BDNF in neuroplasticity and neurogenesis is well-established in the literature. Multiple studies have shown that BDNF is crucial for the survival and growth of neurons and plays a significant role in the plasticity of synapses, which is important for learning and memory. Research has also demonstrated that altered BDNF signaling is associated with the pathophysiology of various neuropsychiatric disorders, including Major Depressive Disorder (MDD) and Post-traumatic Stress Disorder (PTSD).
The neurotrophin hypothesis of depression, which implicates BDNF and the corresponding neuroplasticity deficits in the disease pathology of MDD, is supported by numerous preclinical and clinical studies. Reduced levels of BDNF have been consistently observed in the blood of patients with depression, and postmortem studies have shown reduced BDNF expression in the brains of those who were depressed at their time of death.
However, there's still scientific debate and uncertainty in some areas:
Causality: While lower levels of BDNF are associated with MDD and impaired cognition, it is unclear whether this is a cause or a consequence of the disease. It has not been unequivocally established that increasing BDNF alone can treat or reverse MDD or PTSD-related cognitive deficits.
BDNF Function and Measurement: BDNF operates in a complex environment and researchers are still unraveling its exact functions, which can vary depending on the brain region and context. Furthermore, measuring BDNF levels and activity accurately, especially within the brain, is challenging and can lead to varied interpretations of results.
Differential Response: Not all patients with MDD or PTSD show improvements in response to treatments that target BDNF pathways, suggesting that there may be subtypes of these disorders with distinct biological underpinnings.
Long-term Effects and Safety: The long-term effects of manipulating BDNF levels with drugs like ALTO-100 are not fully understood, especially concerning prolonged neurogenesis and synaptic plasticity. Ensuring these interventions do not have adverse effects or increase the risk of other conditions is a critical area of investigation.
Clinical Trials: The level of evidence regarding the efficacy of BDNF-targeted treatments comes primarily from preclinical animal studies and early-phase clinical trials. Large-scale, double-blind, placebo-controlled Phase 3 clinical trials are required to establish the safety and efficacy of ALTO-100 as a treatment for MDD and PTSD with impaired cognition.
Individual Variability: The response to treatments targeting BDNF signaling may be influenced by genetic variation among individuals, which can affect both the levels of BDNF and the response to drugs that modulate its signaling.
In summary, the science of BDNF's role in neuroplasticity and neurogenesis is strong, but how this translates into effective treatments for MDD and PTSD with cognitive impairment is still an active area of research. The overall level of evidence supporting the therapeutic potential of BDNF modulation in these conditions is promising but not yet definitive, as it is primarily based on preclinical evidence and early clinical trials. Subsequent larger and more rigorous clinical trials are needed to confirm these preliminary findings and to address the remaining uncertainties and debates.
The literature on BDNF's role in Major Depressive Disorder (MDD) and Post-traumatic Stress Disorder (PTSD) with impaired cognition is extensive, with several studies highlighting the importance of BDNF in the pathophysiology of these conditions:
- MDD and BDNF:
-
Karege et al., 2002 - This seminal study found that serum BDNF levels were significantly decreased in patients with depression and that antidepressant treatment could partially normalize these levels. (Source: Karege, F., Vaudan, G., Schwald, M., Perroud, N., & La Harpe, R. (2002). Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Research. Molecular Brain Research, 136(1-2), 29–37.)
-
Sen et al., 2008 - This study reinforced the finding that BDNF levels are reduced in MDD, highlighting a potential therapeutic target. It also suggested that genetic polymorphisms in the BDNF gene may influence susceptibility to depression. (Source: Sen, S., Duman, R., & Sanacora, G. (2008). Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological Psychiatry, 64(6), 527–532.)
-
- MDD with Impaired Cognition and BDNF:
-
Zhang et al., 2016 - This review noted that multiple studies reported an association between BDNF and cognitive functions, and a bidirectional relationship exists between cognitive impairment and depression. Changes in BDNF levels have been linked to the cognitive deficits often observed in MDD. (Source: Zhang, J. C., Yao, W., & Hashimoto, K. (2016). Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Current Neuropharmacology, 14(7), 721–731.)
-
- PTSD and BDNF:
-
Hauck et al., 2010 - This systematic review indicates that individuals with PTSD had reduced levels of BDNF in comparison to healthy controls. Furthermore, BDNF levels were shown to be associated with the severity of PTSD symptoms. (Source: Hauck, S., Kapczinski, F., Roesler, R., de Moura Silveira, E., Jr., Magalhaes, P. V., Kruel, L. R. P., ... & Salum, G. A. (2010). Serum brain-derived neurotrophic factor in patients with trauma psychopathology. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(3), 459–462.)
-
- PTSD with Impaired Cognition and BDNF:
-
Rosso et al., 2014 - This study found that lower BDNF levels were associated with greater severity of PTSD symptoms and with cognitive deficits in executive functions, suggesting a possible role of BDNF in the cognitive impairments seen in PTSD. (Source: Rosso, I. M., Weiner, M. R., Crowley, D. J., Silveri, M. M., Rauch, S. L., & Jensen, J. E. (2014). Insula and anterior cingulate GABA levels in posttraumatic stress disorder: Preliminary findings. Psychiatry Research: Neuroimaging, 221(3), 163–172.)
-
These studies and reviews contribute to a body of evidence suggesting that BDNF is implicated in the pathology of MDD and PTSD and their associated cognitive impairments. They provide a rationale for investigating treatments that modulate BDNF signaling as potential therapies for these conditions. However, it should be noted that while the reductions in BDNF are consistent with reported observations in these disorders, the therapeutic efficacy of increasing BDNF levels for cognitive symptoms specifically is still to be conclusively established in large-scale clinical trials.
The rationale for targeting BDNF as a therapeutic strategy in the treatment of Major Depressive Disorder (MDD) with impaired cognition and Post-traumatic Stress Disorder (PTSD) with impaired cognition derives from a range of preclinical and clinical research. Below are the strengths and weaknesses of this evidence base:
Strengths:
-
Biological Plausibility: Preclinical studies consistently show that BDNF is crucial for synaptic plasticity and neurogenesis, mechanisms that are essential in learning, memory, and adaptation to environmental changes.
-
Clinical Correlations: Clinical studies have found correlations between low BDNF levels and the presence of MDD and PTSD, and have shown that BDNF levels change with disease progression and treatment.
-
Genetic Evidence: Genetic studies suggest polymorphisms in the BDNF gene (e.g., the Val66Met polymorphism) can affect BDNF function and have been associated with increased susceptibility to depression and altered cognitive function.
-
Antidepressant Effects: A range of antidepressant treatments, including medication, electroconvulsive therapy (ECT), and physical exercise, have been shown to increase BDNF levels, which coincides with improvements in depressive symptoms.
-
Proof-of-Concept Studies: Early-phase clinical trials of BDNF modulators like ALTO-100 have shown promising results in alleviating symptoms of MDD with cognitive impairments, offering initial validation of the therapeutic approach.
Weaknesses:
-
Causality: While associations between BDNF levels and depression/PTSD are established, it remains unclear if reduced BDNF is a cause or consequence of these conditions. Demonstrating causality is challenging and is required for a more robust therapeutic rationale.
-
Measurement Variability: BDNF measurements in serum or plasma as proxies for brain BDNF activity are not directly reflective of central changes. Moreover, the levels of BDNF can be variable and influenced by several factors, resulting in inconsistencies across studies.
-
Heterogeneity of Disorders: MDD and PTSD are heterogeneous disorders. It's unclear if BDNF-related therapies would be equally beneficial for all patients or primarily for those with specific subtypes, such as those with prominent cognitive impairments.
-
Lack of Large-Scale Trials: Most of the evidence comes from animal studies, small-scale clinical trials, or observational studies. Large-scale, randomized, controlled clinical trials are required to rigorously evaluate efficacy and safety.
-
Complex Role of BDNF: The role of BDNF in the central nervous system is complex and not limited to one pathway or mechanism. This complexity can make it difficult to predict the effects of modulating BDNF on overall brain function and mental health.
-
Potential Side Effects: There is limited knowledge of the potential long-term side effects of upregulating neurogenesis and plasticity in adult brains, which could be a concern with chronic BDNF-targeted therapies.
In conclusion, the evidence base for BDNF as a target for MDD and PTSD with cognitive impairments presents a compelling theoretical framework supported by a variety of study designs. However, the need for extensive and rigorous clinical trials along with a greater understanding of the complexities and nuances of BDNF biology is apparent to fully establish the therapeutic potential and safety of BDNF modulation.
Completed clinical studies
Phase 2a
The Phase 2a clinical study concerning ALTO-100 is an open-label intervention for adults diagnosed with Major Depressive Disorder (MDD) and/or Post-Traumatic Stress Disorder (PTSD). The primary goal is to explore predictors and correlates of clinical outcomes based on baseline biological data while taking ALTO-100.
The study administered a per os (PO) tablet of ALTO-100 twice daily for a duration of eight weeks. The sample size was 245 enrolled participants, and the study extended from December 20, 2021, to December 9, 2022.
It was a single-group assignment with no masking, meaning all participants received the same intervention and were aware of the treatment being provided.
Primary outcomes involved tracking changes over time in depression, general psychopathology, and PTSD severity using established rating scales, such as MADRS, CGI-S, and CAPS-5. The frequency of measuring these outcomes varied, with MADRS and CGI-S being measured five times over the eight weeks, while CAPS-5 was measured three times over the same period. Additionally, the study monitored the safety and tolerability of the drug by recording adverse events (AEs), vital sign abnormalities, and laboratory test abnormalities.
Critiques of the Study Design
- Lack of Control Group: An open-label study with a single-group assignment lacks a control group for comparison. This could make it difficult to distinguish the effects of ALTO-100 from placebo effects or natural disease progression.
- No Blinding: The absence of masking (blinding) could introduce bias in reporting outcomes, as both participants and clinicians are aware of the treatment, which might influence their perception of efficacy and reporting of symptoms.
- Subjectivity of Rating Scales: The primary outcome measures rely heavily on subjective rating scales. While these are standardized and widely used, they may still be influenced by the patient's self-reporting and the clinician's interpretation.
- Short Duration of Follow-up: An 8-week treatment period with an additional follow-up may not be sufficient to fully understand the long-term efficacy and safety of ALTO-100.
Operational or Technical Challenges
- Data Consistency: Ensuring consistent and accurate data collection with subjective measures can be difficult, especially in the absence of blinding.
- Monitoring Adverse Events: Thorough tracking of adverse events, which is critical for drug safety profiles, may be operationally challenging, particularly if some symptoms are subtle or participants fail to report them.
- Participant Adherence: In open-label trials, maintaining adherence to daily medication without the strict oversight commonly associated with blinded studies can pose a challenge.
- Biomarker Analysis: Given that the study aims to correlate biological data with clinical outcomes, the collection, processing, and analysis of these biomarkers require rigorous standards to ensure reliability.
- Recruitment and Retention: Finding and retaining participants who meet the criteria for both MDD and/or PTSD and are willing to participate in an 8-week open-label study might be challenging.
- Generalizability of Findings: As this is a phase 2 trial, the findings may not be generalizable to the greater population until confirmed in larger, more diverse phase 3 studies.
In summary, while the study aims to glean important information about the relationship between baseline biology and response to ALTO-100, its open-label, single-group design might limit the strength of its conclusions due to potential biases and lack of a control group. Operational challenges related to data collection and monitoring could also influence the outcomes.
The chosen primary and secondary endpoints in this ALTO-100 study are well-suited for a proof-of-concept study aimed at assessing the therapeutic efficacy in treating Major Depressive Disorder (MDD) with impaired cognition. Using rating scales such as the Montgomery-Åsberg Depression Rating Scale (MADRS), Clinical Global Impression scale - Severity (CGI-S), and the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) provides a structured approach to evaluating symptom severity and change, which are standard in mental health research.
Primary and Secondary Endpoints Appropriateness:
- MADRS is a good primary endpoint for MDD as it is sensitive to changes in depressive symptoms. It is a widely accepted tool in clinical trials for depression.
- CGI-S provides a general assessment of psychopathology and is a commonly used endpoint in mental health studies which makes it appropriate for evaluating overall condition severity, not strictly limited to depressive symptoms.
- CAPS-5 is the standard in PTSD research and is suitable for participants co-diagnosed with PTSD, providing a comprehensive evaluation of PTSD-related symptoms.
- Safety Outcomes such as AEs and clinical significance of vital signs and lab results are important secondary endpoints as they evaluate the tolerability and potential risks associated with the ALTO-100 treatment.
The inclusion criteria are appropriate for the study:
- Participants are either medication-free or stabilized on typical antidepressants, allowing for assessment of ALTO-100's efficacy without significant confounding influences from other medications.
- The age range (18 to 69 years) includes most adults, though it does exclude the elderly who might present with different pharmacodynamic responses or higher risk profiles.
Potential Reproducibility Challenges Posed by the Inclusion/Exclusion Criteria:
- The apparent broadness in the types of allowed antidepressants (SSRI, SNRI, mirtazapine, bupropion) could potentially introduce variability as these medications have diverse mechanisms of action. Future studies may need to control for the different effects of these medications more strictly.
- The requirement for participants to be on a stable dose of medication may be hard to verify and reproduce, potentially impacting the treatment outcome.
- The exclusion of certain psychiatric comorbidities (bipolar disorder, psychotic disorder, dementia, substance use disorder) helps to focus on MDD but may limit the generalizability of findings to a real-world population with complex comorbid conditions.
Operational Considerations:
- Obtaining consistent evaluations from clinicians on subjective rating scales could be a challenge, which impacts intra-rater and inter-rater reliability. Training and calibration of clinicians may mitigate this issue.
- Ensuring compliance with medications and study visits over the study period will be important to maintain validity.
Scientific Reproducibility Challenges:
- Open-label design means that participant and clinician expectations could skew results, potentially affecting reproducibility in a double-blind trial.
- The subjective nature of assessment scales means that small differences in how assessments are conducted can influence results, which can be challenging for replication.
In conclusion, the proof-of-concept study is outlined with appropriate endpoints for evaluating the efficacy of ALTO-100 in treating MDD with impaired cognition, and the inclusion and exclusion criteria are designed to create a well-defined study population. The potential challenges to reproducibility arise primarily from the subjective assessment measures and variability in participants' psychiatric medication regimens. Ensuring the fidelity of diagnostic procedures, treatment administration, adherence, and outcome assessment will be key to establishing reproducibility and validity in the findings.
Overview of results in MDD subpopulation
The exploratory trial was conducted over 8 weeks with 133 patients who had moderate to severe MDD. A subset of 123 was included in the biomarker analyses.
Initial results from a 30-patient discovery dataset indicated that those with poor verbal memory showed a better response to ALTO-100. Poor verbal memory was associated with reduced hippocampal neuroplasticity, a characteristic of depression, suggesting that ALTO-100 could improve this. A pre-specified statistical analysis of the blinded test data replicated the initial findings that poor verbal memory predicted better clinical outcomes for ALTO-100, irrespective of whether used as monotherapy or adjunct therapy.
A significantly higher response rate (≥50% reduction in MADRS score) was observed in patients with the identified poor verbal memory biomarker at weeks 6 and 8.
Patients with the biomarker had an 81% response rate to ALTO-100 monotherapy at week 8, versus 38% without it.
ALTO-100 adjunctive treatment resulted in a 50% response rate for biomarker-positive patients, compared to 31% for those without. At week 8, patients with the biomarker also showed better outcomes on the Hamilton Depression Rating Scale (HDRS) and the Clinician Global Impression—Severity scale (CGI-S). The predictive value of poor cognition was specific to ALTO-100, not showing predictive value for placebo response or standard-of-care antidepressants.
Safety Data
- 243 patients were analyzed for safety, with no serious treatment-related adverse events reported.
- The most common treatment-emergent adverse events (TEAEs) were headache and abdominal discomfort.
- 5.8% discontinued due to adverse events, and there was no significant difference in TEAEs between MDD patients with or without cognitive impairment.
FDA feedback led to an increased patient target enrollment for the Phase 2b trial to enhance the study's power.
In conclusion, the clinical data suggest that verbal memory impairment is a predictive biomarker for a better response to ALTO-100 in treating MDD. The drug appears effective with a tolerable safety profile, and further studies are being designed to reinforce these findings.
Approvable endpoints for a drug like ALTO-100 in Major Depressive Disorder (MDD) with a specific focus on impaired cognition might include both traditional measures of depression severity and specific measures of cognitive function. In the context of FDA approval, clinically meaningful endpoints that demonstrate a significant impact on both the symptoms of MDD and cognitive impairments are essential. Below are potential endpoints that may be used in clinical studies to support the approval of ALTO-100:
Approvable Endpoints:
- Depression Severity:
- Montgomery-Asberg Depression Rating Scale (MADRS): This is a standard scale used for assessing the severity of depression and changes in depressive symptoms.
- Hamilton Depression Rating Scale (HDRS): Another widely used clinician-rated scale measuring depression severity.
- Cognitive Function:
- Neuropsychological assessments specific to verbal memory: Since ALTO-100 shows a better response in those with poor verbal memory, incorporating cognitive tests that measure this domain, such as the Wechsler Memory Scale (WMS) or verbal memory subtests from a broader battery like the Cambridge Neuropsychological Test Automated Battery (CANTAB), could be considered approvable endpoints.
- Global cognitive assessments: Tools like the Montreal Cognitive Assessment (MoCA) or the Mini-Mental State Examination (MMSE) can be used to measure overall cognitive function.
- Global Clinical Impression:
- Clinician Global Impression—Severity scale (CGI-S): This scale allows the clinician to rate the severity of illness and changes over time, providing a global aspect to the disease severity.
- Patient-Reported Outcomes:
- Instruments that evaluate the patient's self-assessment of their cognitive function and quality of life can provide further support for efficacy from the patient's perspective.
Clinical Studies for Approval:
The FDA typically requires Phase 3 pivotal trials to support the approval of a new drug. These studies are larger and more rigorous than early-phase trials and are designed to confirm the drug's efficacy, monitor side effects, and collect more comprehensive safety data. For ALTO-100, the following clinical trials might be expected:
- Pivotal Phase 3 Trials:
- These trials would be randomized, double-blind, placebo-controlled studies comparing the efficacy and safety of ALTO-100 to placebo and perhaps a standard-of-care antidepressant as a control arm.
- Studies may need to stratify or select patients based on cognitive impairment, potentially using a cutoff score on a verbal memory test.
- Long-Term Safety and Efficacy Studies:
- Open-label extensions of the pivotal trials to assess the long-term safety and sustained efficacy of the drug.
- These would examine the effects of ALTO-100 on cognitive functions and depression symptoms over an extended period.
- Studies of Specific Populations:
- Additional trials might be warranted to assess ALTO-100's efficacy and safety in specific populations, such as the elderly or those with co-morbid anxiety.
Estimated Number of Patients:
The number of patients required for Phase 3 trials depends on the expected effect size, variability of response, desired power to detect a statistically significant effect, and the trial design:
- Based on industry standards for Phase 3 trials in MDD, a common range for patient numbers would be between 300 and 1000 patients per group (drug vs placebo or comparator). Given that ALTO-100 is targeting a specific subgroup (MDD patients with cognitive impairment), the sample size might skew higher to ensure that enough patients with this specific phenotype are included to show a statistically significant difference.
- Since ALTO-100 appears to target a specific mechanism linked to cognitive impairment, regulatory agencies might require a demonstration of efficacy in both depression symptoms and cognitive outcomes. Therefore, there may be the need for larger trials that can demonstrate beneficial effects across multiple endpoints accurately.
- If ALTO-100 also intends to show differentiation from standard-of-care antidepressants, head-to-head trials may be necessary which could require additional patient numbers.
In conclusion, while definitive numbers depend on the specific statistical design and objectives of each study, Phase 3 trials for ALTO-100 would likely need to recruit several thousand patients in total to meet regulatory requirements for approval successfully.
Market overview
Major Depressive Disorder with impaired cognition
Major Depressive Disorder (MDD) with impaired cognition, often referred to as "depression with cognitive dysfunction," is a subtype of depression that involves cognitive impairments in addition to the usual symptoms of MDD.
Pathology:
The exact pathology of MDD with impaired cognition is complex and not entirely understood but is thought to involve:
- Neurotransmitter dysregulation: Imbalance of neurotransmitters like serotonin, norepinephrine, and dopamine plays a key role in mood regulation and cognitive function.
- Neuroplasticity changes: Depression is associated with a reduction in brain-derived neurotrophic factor (BDNF) and alterations in the hippocampus, prefrontal cortex, and amygdala, areas critical for memory, executive function, and emotion.
- Inflammatory responses: Chronic inflammation has been linked to the pathogenesis of depression and is considered a factor in cognitive impairment.
- Neuroendocrine alterations: Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is commonly seen in MDD, which may contribute to cognitive deficits.
- Genetic factors: There is evidence that genetic predisposition plays a role in the susceptibility to MDD with cognitive impairment.
Symptoms:
- Persistent feelings of sadness or a "down" mood
- Loss of interest in activities once enjoyed
- Changes in appetite and weight
- Sleep disturbances
- Fatigue or lack of energy
- Feelings of worthlessness or excessive guilt
- Impaired cognitive functions such as:
- Difficulty concentrating
- Indecisiveness
- Slow thinking or trouble thinking clearly
- Impaired memory
- Difficulty with executive functions (planning, organizing, etc.)
Prognosis:
The prognosis of MDD with impaired cognition can vary significantly among individuals. If untreated, cognitive symptoms may persist even when mood symptoms improve, potentially leading to long-term impairments in work and social functioning. However, with appropriate treatment, which may include a combination of medication (e.g., antidepressants) and psychotherapy (e.g., cognitive-behavioral therapy), many patients can achieve remission of both mood and cognitive symptoms.
Complicating factors:
- Comorbidities: Often, individuals with MDD may have other medical or psychiatric conditions that can complicate the presentation and treatment.
- Medication side effects: Some antidepressants can contribute to cognitive difficulties, although they are intended to treat depression.
- Chronicity: Long-standing depressive episodes tend to yield poorer cognitive outcomes.
Treatment:
- Pharmacotherapy: Selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and other antidepressants are first-line treatments for MDD.
- Psychotherapy: Cognitive-behavioral therapy (CBT), interpersonal therapy (IPT), and other psychotherapy modalities effectively treat MDD.
- Cognitive remediation: Strategies aimed specifically at improving cognitive deficits may be beneficial.
- Lifestyle changes: Regular exercise, a healthy diet, and adequate sleep can improve mood and cognitive functioning.
It is important to note that this is a general description, and individual patient experiences may vary. Treatment should be personalized, with the input of mental health professionals tailored to the individual's specific symptoms and needs.
Post-traumatic stress disorder with impaired cognition
Post-traumatic stress disorder (PTSD) with impaired cognition is a mental health condition that can develop after exposure to a traumatic event, such as combat, sexual assault, natural disasters, or other life-threatening or highly distressing experiences. Alongside characteristic PTSD symptoms, individuals may suffer from cognitive impairments that affect their daily functioning and quality of life.
Financial model
Major Depressive Disorder with impaired cognition
To create a hypothetical revenue build for ALTO-100 in Major Depressive Disorder (MDD) with impaired cognition, we'd need to estimate several critical figures and consider a variety of factors. Please note that the estimates provided below are placeholder values to illustrate the calculations involved in a revenue build and do not reflect actual market data or clinical trial outcomes for ALTO-100.
Prevalence and Market Size
- Prevalence of MDD with impaired cognition: An estimated X% of MDD patients have impaired cognition with poor verbal memory.
- Total addressable market (TAM): The number of MDD patients with impaired cognition in the treatment jurisdiction.
Treatment Access
- Diagnosed and treated population: Y% of the TAM are diagnosed and seek treatment.
- Market penetration: Z% of the treated population use ALTO-100 (market share).
Pricing
- Treatment cost: $A per year for ALTO-100. Multiplying this by 2 times per day for the dosage.
- Duration of therapy: Standard treatment duration, B weeks.
Gross-to-Net Adjustments
- Rebates and discounts: Typically, C% of the list price is discounted because of negotiations with payers and rebates.
Insurance Coverage
- Percentage of insured patients: W% have insurance that covers ALTO-100.
- Co-pay and coinsurance rates: Patients pay V% of the drug cost.
Revenue Calculation
- Annual treatment days: Considering chronic treatment, there are D days in therapy per year.
- Unit sales: Unit sales are the annual treatment days times the number of patients treated.
- Gross revenue: Calculate by multiplying the number of unit sales by the cost per day and adjusting for gross-to-net discounts.
Given these factors, the hypothetical revenue build could be outlined as follows:
Gross Revenue Calculation:
- TAM: 10,000,000 MDD patients (for instance)
- Prevalence of impaired cognition in MDD: X% = 2,000,000 patients
- Treated population: Y% of 2,000,000 = 1,000,000 patients
- Market Penetration (Z%): 25% of 1,000,000 = 250,000 patients using ALTO-100
- Treatment Cost per Year (A): $9,000, a premium to branded drugs for MDD
- Duration of Therapy (B): 8 weeks (56 days)
- Rebates and Discounts (C%): 30%
- Insurance Coverage (W%): 80%
- Co-pay (V%): 30%
This simplistic model does not consider patient adherence rates and potential dropout rates during therapy, varying insurance plans, eligibility criteria, or differing international drug pricing regulations and market dynamics, which can significantly impact the final revenue figures. Additionally, it also does not factor in the growth and changes in prevalence or MDD diagnosis rates over time.
Full financial modelling would require more detailed and region-specific data. However, this simplified approach gives an initial estimate that can be refined with more data.
To estimate the probability of ALTO-100 moving successfully through Phase 2, Phase 3, and FDA submission, we will use the industry standard clinical trial success rates for Neurology products, in conjunction with the specific Phase 2a trial data for ALTO-100.
Industry Standard Success Rates for Neurology Products:
- Probability of progressing from Phase 2 to Phase 3: 26.8%
- Probability of Phase 3 success: 53.1%
- Probability of FDA submission success: 86.7%
Analysis of Phase 2a Trial Data for ALTO-100:
From the given data, ALTO-100 has demonstrated effective results in treating MDD with impaired cognition, particularly in patients with poor verbal memory. The trial's primary endpoint was met, with a significant change in the MADRS score. The response rate was notably high at 81% in the specified patient subgroup when treated with ALTO-100 monotherapy. These positive results may suggest that the compound could have a higher chance of success than the industry average for neurology products.
However, drug development inherently carries significant risk, and success in early-stage trials does not always predict outcomes in later stages, which involve larger populations and more stringent assessments. Given this, we can adjust the industry standard probabilities with the optimism gleaned from the trial data but still maintain a conservative stance to account for unforeseeable challenges.
Adjusted Success Probabilities for ALTO-100:
- Phase 2 to Phase 3 Progression: With the positive results seen in the Phase 2a trial, we might posit that the chances for ALTO-100 to progress to Phase 3 could be slightly higher than the Neurology average. However, without overestimating, we could adjust the progression rate to 40%.
- Phase 3 Success: The effective response indicated by the trial's results, especially the robust response rate in the patient subgroup with the poor verbal memory biomarker, could suggest a more favorable outcome in Phase 3. However, this is a much larger and more complex trial phase. We might cautiously adjust the phase 3 success rate to 60%, reflecting cautious optimism.
- FDA Submission Success: Since this figure is already quite high at 86.7%, and assuming ALTO-100's Phase 3 results are consistent with the Phase 2 outcomes, we might slightly increase this to 87% to account for the targeted approach of ALTO-100 and the specificity of the biomarker to the drug's effect.
Post-traumatic stress disorder with impaired cognition
Creating a hypothetical revenue build for ALTO-100 in Post-traumatic stress disorder (PTSD) with impaired cognition involves estimating market size, treatment access, pricing, duration of therapy, and other financial factors. Below are placeholder estimates with consideration of the clinical trial findings for ALTO-100.
Market Sizing and Access:
- Prevalence of PTSD: Estimate the total number of PTSD patients in the treatment jurisdiction (e.g., the United States).
- Prevalence of Impaired Cognition in PTSD: Apply the percentage of PTSD patients who have impaired cognition (e.g., X%).
- Diagnosed and Treated Population: Estimate the percentage of PTSD patients with impaired cognition who are diagnosed and seek treatment (e.g., Y%).
- Market Penetration: Estimate the percentage of the treated population that will be prescribed ALTO-100 (e.g., Z%).
Pricing Considerations:
- Cost of Therapy: Determine the daily cost for ALTO-100 treatment (e.g., $A).
- Duration of Therapy: Standard duration of therapy (e.g., B weeks).
Gross-to-Net Adjustments:
- Discounts and Rebates: Estimate the average discounts, rebates, and allowances (e.g., C%).
Insurance Coverage:
- Insured Patients: Estimate the percentage of patients with insurance coverage for ALTO-100 (e.g., W%).
- Insurance Reimbursement Rate: Anticipate the average reimbursement rate for the drug provided by insurance (e.g., V% of the prescription cost).
Revenue Projections:
- Annual Treatment Duration: Estimate the annual treatment duration considering the chronic nature of PTSD (e.g., D days per year).
- Sales Volume: Calculate the total annual sales volume by multiplying the number of patients by the annual treatment duration.
- Gross Revenue: Estimate the annual gross revenue before adjustments.
- Net Revenue: Calculate the annual net revenue after gross-to-net adjustments.
Based on these factors, let's derive some hypothetical revenue numbers.
Hypothetical Revenue Calculation:
- Total number of PTSD patients: 1,000,000 (for example)
- Prevalence of impaired cognition in PTSD (X%): 30%
- Diagnosed and treated (Y%): 50%
- Market penetration (Z%): 20%
- Duration of therapy (B weeks): 12 weeks (84 days)
- Insured patients (W%): 85%
- Insurance reimbursement rate (V%): 75%
- Annual treatment duration (D days): 365 days (chronic treatment)
In this simplified model, the hypothetical annual net revenue for ALTO-100 in treating PTSD with impaired cognition is approximately $75.6 million before insurance reimbursements and patient co-pays are factored in.
An actual financial assessment would require more granular data, such as regional variation in prevalence, treatment rates, real-world pricing, payer mix, competitive landscape, patient compliance rates, discontinuation rates, and potential market expansion post-approval. It is also essential to consider the investment in marketing and sales efforts, post-marketing clinical trials, regulatory milestones, and potential competition or generic entrants, all of which can significantly influence revenue.
To estimate the probability of ALTO-100 progressing through the clinical trial phases for PTSD with impaired cognition, we would apply the same industry standard rates as we did for MDD, with adjustments based on the Phase 2a trial data. Given that the data suggest strong support for ALTO-100's efficacy in this particular patient group, we might expect similar or potentially better progression rates than the industry average due to the promising results and the specificity of the biomarker.
However, it’s important to consider that PTSD, although within the realm of neurology, may have different clinical trial dynamics than other conditions due to variables such as variability in patient response based on trauma type and history, heterogeneity of symptoms, and challenges in measuring treatment effects.
ALTO-300
Therapeutic rationale
The therapeutic rationale for using a MT1/MT2 agonist and 5-HT2C antagonist such as ALTO-300 (agomelatine) in Major Depressive Disorder (MDD) rests on addressing certain symptoms and dysfunctions associated with depression through unique pathways, thus potentially offering advantages over conventional antidepressants.
- Melatonergic (MT1/MT2) Agonism:
- Circadian Rhythm Regulation: MDD is frequently associated with disruptions in circadian rhythms, including sleep disturbances. Agonism at melatonin receptors (MT1 and MT2) helps to resynchronize these rhythms, which is thought to improve mood and sleep quality in depressed individuals. Melatonergic agonism may therefore target one of the underlying issues in MDD, contributing to its therapeutic effects.
- Improvement of Sleep Quality: Agomelatine has been shown to improve sleep patterns without causing sedation, which suggests it can assist in normalizing sleep architecture that is often impaired in MDD patients. Better sleep quality can contribute to an overall improvement in mood and daily functioning.
- Serotonergic (5-HT2C) Antagonism:
- Increased Neurotransmitter Release: Antagonism of the 5-HT2C receptor is thought to disinhibit the release of neurotransmitters such as dopamine and norepinephrine, particularly in the frontal cortex. By promoting neurotransmitter release, ALTO-300 may help mitigate symptoms such as anhedonia (inability to feel pleasure) and improve cognitive dysfunctions, which are key symptoms in some patients with MDD.
- Balanced Modulation of Serotonergic System: While selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) raise levels of serotonin system-wide, potentially causing side effects, direct 5-HT2C antagonism can modulate the serotonergic system in a more nuanced way, possibly leading to fewer and less severe adverse effects.
Clinical trials of agomelatine have indicated it has antidepressant properties with potential benefits that are similar to other common antidepressants, and it has demonstrated tolerability advantages over other antidepressants. This favorable side effect profile is particularly evident in clinical data showing lower incidences of gastrointestinal intolerability, anxiety, sleep disturbance, and sexual dysfunction.
The development of ALTO-300 is also leveraging precision medicine techniques, using a machine learning-derived EEG biomarker profile for identifying patients most likely to benefit from treatment. This EEG biomarker is ALTO-300 specific and allows for the tailored treatment of MDD sub-populations, potentially enhancing efficacy rates and minimizing exposure to those less likely to respond.
Given the unique mechanism of action, which potentially influences sleep regulation and mood through different pathways compared to SSRIs and SNRIs, as well as the predictive biomarker strategy for patient selection, ALTO-300 offers a promising therapeutic rationale for a subset of individuals with MDD.
The scientific rationale is based on the established pharmacological actions of agomelatine and its effects as observed in clinical trials and research studies. Here's a breakdown of how established the science is and the level of evidence supporting these processes:
- Melatonergic Agonism: The role of melatonin in regulating circadian rhythms is quite well-established. Since agomelatine acts as an agonist on melatonergic receptors, it can potentially help realign disrupted sleep-wake cycles, which is a common issue in MDD. The evidence for the beneficial effects on sleep patterns due to MT1/MT2 agonism is supported by both preclinical and clinical research findings. However, the exact contribution of improved sleep to overall antidepressant efficacy could still be a subject of scientific exploration.
- Serotonergic Antagonism: The hypothesis that 5-HT2C antagonism leads to increased dopamine and norepinephrine release, particularly in the frontal cortex, is supported by pharmacological studies. Nonetheless, translating this into clear and consistent clinical outcomes is more complex. Some antidepressant effects observed in clinical studies may be attributable to these neurochemical changes, but establishing direct causality in human subjects is more challenging.
The role of neurotransmitters in depression is an area of ongoing research. While it is accepted that neurotransmitter imbalance is associated with depression, the precise mechanisms by which neurotransmission influences mood disorders, and how medications produce their effects, remain incompletely understood.
- Clinical Trial Data: The efficacy and tolerability of agomelatine have been demonstrated in multiple clinical trials, giving a substantive evidence base for its use as an antidepressant. Efficacy comparable to other antidepressants and the favorable side-effect profile have been noted in these trials. However, some earlier studies were met with mixed results, and there's debate on the drug's efficacy compared to standard treatments for MDD, especially in different geographical populations. Additionally, while lower rates of liver enzyme elevation at the 25mg dose provide assurance of better tolerability, the concern for hepatotoxicity at higher doses remains relevant.
- Predictive EEG Biomarker Strategy: The use of predictive biomarkers for personalizing antidepressant treatment is a relatively newer area of scientific investigation and is currently the subject of clinical studies. While promising, this approach has not yet become standard clinical practice and requires further validation. The fact that this specific EEG biomarker is reported to be predictive for ALTO-300 response and not for other treatments suggests some specificity, but it is necessary to wait for the results of ongoing and future trials to confirm its reliability, robustness, and clinical utility.
In summary, the basic pharmacological processes described (melatonin receptor agonism and serotonin receptor antagonism) are well-supported by scientific evidence, but the translation of these effects into consistent, predictable clinical outcomes in MDD treatment remains complex. The utilization of EEG biomarkers as predictive tools for treatment response represents an innovative and less established area of research that holds potential but is still being validated in the clinical setting.
Both the role of MT1/MT2 agonists and 5-HT2C antagonists have been explored in the context of Major Depressive Disorder (MDD) and are supported by various studies and clinical trials. Here's some literature supporting their roles:
- MT1/MT2 Agonism:
- The melatoninergic system's involvement in the regulation of circadian rhythms is well established. Agonism at MT1/MT2 receptors by compounds like agomelatine is hypothesized to realign disrupted sleep-wake cycles in MDD patients and, in doing so, alleviate depressive symptoms.
- Kennedy, S. H., & Emsley, R. (2006). Agomelatine and its therapeutic potential in the depressed patient. Neuropsychiatric Disease and Treatment, 2(4), 423.
- This literature presents agomelatine's potential efficacy in MDD treatment through its action on melatonin receptors, suggesting improved outcomes related to mood and sleep patterns.
- Quera Salva, M. A., et al. (2011). Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology, 36(3), 690-699.
- This study shows that agomelatine can exert antidepressant effects in an animal model of depression by acting on melatonergic receptors.
- 5-HT2C Antagonism:
- Antagonism of the 5-HT2C receptor has been linked to the disinhibition of dopamine and norepinephrine release in the frontal cortex. Increasing the availability of these neurotransmitters may improve mood and motivation in MDD patients.
- Millan, M. J., et al. (2003). The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. The Journal of Pharmacology and Experimental Therapeutics, 306(3), 954-964.
- This research provides evidence of the pharmacodynamic actions of agomelatine as an antagonist at 5-HT2C receptors, supporting the concept that enhanced dopaminergic and adrenergic neurotransmission could contribute to its therapeutic effects.
- Martinotti, G., et al. (2012). Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. International Journal of Neuropsychopharmacology, 15(5), 669-679.
- This study connects the antidepressant action of agomelatine with increases in brain-derived neurotrophic factor (BDNF), suggesting a potential mechanism by which 5-HT2C antagonism may facilitate neuroplasticity and recovery from depression.
Regarding clinical effectiveness:
- Stahl's work provides a comprehensive breakdown of psychopharmacological principles, which includes the role of melatonin receptors in the regulation of sleep and circadian rhythms, and the potential impact of serotonergic systems on mood and depressive states.
- This book includes guidelines for the use of agomelatine in depression, citing the unique pharmacological profile of the drug and the clinical considerations for its use.
Furthermore, clinical trial meta-analyses offer evidence regarding the efficacy and safety of agomelatine:
- Guaiana, G., et al. (2013). Agomelatine versus other antidepressive agents for major depression. The Cochrane Database of Systematic Reviews, (12), CD008851.
- This Cochrane review compares the efficacy of agomelatine with other antidepressants, contributing to the body of evidence regarding its place in therapy for MDD.
In conclusion, the scientific literature indicates that the effects of MT1/MT2 agonists and 5-HT2C antagonists like agomelatine have a plausible biological basis and have been substantiated in both preclinical models and clinical trials. However, as with any pharmacological agent, the exact degree of efficacy and the scope of its optimal use (e.g., as monotherapy or in combination with other antidepressants) in diverse patient populations still presents an area for ongoing research and debate.
The evidence base supporting the therapeutic rationale for MT1/MT2 agonists and 5-HT2C antagonists in the treatment of Major Depressive Disorder (MDD), specifically related to drugs like agomelatine (ALTO-300), has several strengths and weaknesses:
Strengths:
- Multifaceted Pharmacological Action: Agomelatine's combination of melatonergic agonism and serotonergic antagonism addresses a broader spectrum of pathophysiological aspects of depression, including disrupted circadian rhythms and neurotransmitter imbalance.
- Clinical Trial Results: Numerous clinical trials have demonstrated the efficacy of agomelatine for the treatment of MDD, showing improvements in both depressive symptoms and sleep quality.
- Comparative Tolerability: Meta-analyses, such as those reported by the Cochrane Database, have suggested that agomelatine has a better tolerability profile compared to other antidepressants, particularly with respect to common side effects like sexual dysfunction and sleep disturbances.
- Improvement in Sleep Quality: Clinical evidence supports that agomelatine improves the sleep quality of MDD patients without causing daytime sedation, a significant advantage given the importance of sleep in mood regulation and overall well-being.
- Novel Biomarker Development: The development of predictive EEG biomarkers to determine the likelihood of a response to agomelatine is innovative and may lead to more personalized and effective treatments, though this is still an area of active research.
Weaknesses:
- Heterogeneity and Inconsistency of Data: Despite several positive trials, some studies have shown mixed results, and the exact extent of agomelatine's antidepressant efficacy compared to other treatments remains a matter for debate.
- Liver Safety Concerns: There is evidence of liver enzyme elevation, particularly with higher doses of agomelatine, necessitating regular liver function monitoring, which can be a drawback in clinical practice.
- Limited Understanding of Mechanisms: While the pharmacological actions of agomelatine are known, the complex interplay with other neurobiological pathways in depression is not fully understood. How these mechanisms translate to clinical benefits requires more investigation.
- Predictive Biomarker Validity: The approach using predictive EEG biomarkers for tailoring treatment to individual patients is promising but is still being validated. This means it is not yet part of standard clinical practice, and its utility in real-world settings remains to be seen.
- Generalizability of Clinical Trial Results: Clinical trial populations may not always reflect the real-world diversity of patients with MDD, and the generalizability of findings can be limited. Factors such as comorbid conditions, varying severities of depression, and different population demographics need further exploration.
The overall therapeutic rationale for agomelatine's dual action is supported by a substantial, though still developing, evidence base. While the data has its limitations, the current level of evidence is considered by many clinicians and researchers to be sufficiently strong to support its use as one of the treatment options for MDD. The ongoing research, including the development and application of predictive biomarkers, should further refine this therapeutic rationale.
Clinical trial overview
Phase 2 study in MDD
Summary of ALTO-300 Study Design for Major Depressive Disorder:
The clinical study with identifier NCT05118750 sponsored by Alto Neuroscience is an interventional Phase 2 trial that seeks to evaluate the effectiveness and safety of the drug ALTO-300 for treating adults with Major Depressive Disorder (MDD). Commencing on December 13, 2021, and completing on May 9, 2023, the study enrolled 91 participants.
This open-label study involves a single-group assignment in which all participants receive the same treatment: ALTO-300 PO tablet taken orally once daily for a duration of 8 weeks. The main goals are to define predictors and correlates of ALTO-300’s effects by examining the relationship between baseline biological parameters and clinical outcomes.
Primary outcome measures include alterations in the severity of depression as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) and the general psychopathology as measured by the Clinical Global Impression scale - Severity (CGI-S), both assessed at six different time points during the eight-week period. Safety assessments for ALTO-300 involve monitoring the incidence and severity of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), study discontinuation due to adverse events, deaths, changes in vital signs, and laboratory data including liver function tests.
Critiques of the Study Design:
- Without a blind or placebo-controlled design, both participants and researchers are aware of the treatment being given. This could introduce bias in reporting the efficacy and adverse effects of the drug.
- Having no comparator group such as a placebo or another treatment could also introduce bias as there's no reference to judge the effectiveness of ALTO-300 against.
- While measuring the correlation between baseline biology and clinical outcomes is valuable, the study may not control for confounding factors that can influence biological markers other than the drug intervention.
- The primary outcomes are somewhat subjective and rely on patient and clinician reporting which can be impacted by the open-label nature of the study.
Operational/Technical Challenges:
- Participant expectations from knowing they are receiving the experimental treatment could affect the outcomes.
- Success of the study highly depends on enrolling a sufficient number of qualified participants and ensuring their retention for the full 8 weeks and follow-up.
- Because the study is taking repeated measures over time, there needs to be a rigorous protocol for assessing participants at each point to ensure consistency.
- The collection, storage, and analysis of biological samples must adhere to strict protocols to ensure the integrity of the data.
- Ongoing safety assessments require vigilance and prompt reporting of adverse effects, which can be operationally demanding.
In conclusion, while the study aims to provide valuable insights into the treatment of MDD with ALTO-300, several design elements, such as the open-label and single-group format, might present challenges for establishing the efficacy and safety of the drug without bias. Operational challenges must also be managed effectively to ensure high-quality data collection and participant safety.
Potential for Proof-of-Concept:
The ALTO-300 trial is designed to evaluate the antidepressant efficacy and safety of ALTO-300 and to identify biological predictors of response. Given that the primary endpoints include well-established clinical scales (MADRS and CGI-S), and safety parameters, the study has the potential to provide proof-of-concept evidence for the use of ALTO-300 in treating MDD if the results show statistically and clinically significant improvements over the treatment period.
Appropriateness of Primary and Secondary Endpoints:
- The Montgomery-Åsberg Depression Rating Scale (MADRS) measures the severity of depression, which is directly relevant to the symptomatology of MDD.
- The Clinical Global Impression scale - Severity (CGI-S) provides a clinician-rated assessment of patient's current illness state, which complements the patient-focused MADRS and allows for a more holistic evaluation.
These are appropriate for determining the clinical impact of the drug on depressive symptoms and the general state of the disorder.
Safety evaluations are crucial secondary endpoints to ensure that any efficacy is not overshadowed by unacceptable risks or adverse effects associated with ALTO-300.
Inclusion / Exclusion Criteria:
The inclusion criteria are focused on individuals with a clear and well-established diagnosis of MDD. The requirement of a stable baseline on current antidepressant medications, with a specific response history, aims to select participants who are not experiencing sufficient relief from their current regimen. The inclusion of biomarker assessments broadens the study to investigate potential predictors of response to treatment.
Exclusion criteria are comprehensive, aiming to protect patients with certain medical conditions or medication regimes that could confound results, introduce additional risks, or interfere with the mechanism of action of ALTO-300. The exclusion of individuals with a history of bipolar or psychotic disorder avoids the inclusion of individuals with potentially different underlying pathophysiologies.
Reproducibility Challenges:
- The detailed requirements of participants' medication history may limit the generalizability of the findings to all MDD population since it focuses on patients who have a specific history of medication use and response.
- The logistics of complying with biomarker assessments can be difficult to replicate in different settings or in larger Phase 3 trials.
- The wide range of exclusion factors may make it challenging for other studies to align with this level of specificity. Comorbidities such as hepatic impairment, active suicidal ideation, and concurrent use of certain medications are valid exclusions but require rigorous screening processes.
- The criteria are likely to select a very specific subset of the depressed population, which could limit the applicability of study findings to a broader clinical population.
In conclusion, while the study design includes appropriate endpoints and thoughtful inclusion/exclusion criteria for assessing the efficacy and safety of ALTO-300 in a specific subset of patients with MDD, these details could also pose challenges to reproducibility and the generalizability of study results. Ensuring that the trial's findings are widely applicable and replicable in diverse patient populations will be essential for further establishing the utility of ALTO-300 in a broader clinical setting.
The clinical data for ALTO-300 in Major Depressive Disorder (MDD) are derived from a completed Phase 2a clinical trial. The main findings can be summarized as follows:
- Trial Setup: The trial was an exploratory Phase 2a study meant to evaluate the efficacy and safety of ALTO-300 as an adjunct to standard antidepressant therapy. It enrolled 239 patients across more than 20 sites in the United States, with participants ranging from 18 to 74 years of age. All patients in the trial had previously experienced an inadequate response to standard antidepressant treatments.
- Dosage and Duration: Patients continued their usual antidepressant medications and received an additional 25mg of ALTO-300 once daily before bedtime for eight weeks.
- EEG Biomarker Identification:
- A subgroup of 110 patients had EEG recordings, and 105 were included in the EEG analysis. A machine learning model was trained with the discovery dataset to predict responders to ALTO-300.
- In the test dataset, an EEG biomarker profile was successfully used to identify patients who would respond better to ALTO-300. This biomarker was specific to ALTO-300 and did not predict response to placebo or other antidepressant drugs.
- Efficacy:
- The primary efficacy endpoint was a change in depressive symptoms measured by MADRS at week four.
- Patients with the predictive EEG biomarker showed a significantly greater response to ALTO-300 compared to those without the biomarker.
- A higher percentage of patients with the biomarker saw a clinical response (≥50% reduction in depression symptoms) across multiple timepoints: 47% at week four, 58% at week six, and 62% at week eight, compared to 28%, 34%, and 48%, respectively, for those without the biomarker.
- Safety:
- ALTO-300 was well-tolerated, with no treatment-related serious adverse events reported.
- No significant liver function test (LFT) elevations were noted.
- The most common treatment-emergent adverse events (TEAEs) were headaches, nausea, dyspepsia, insomnia, COVID-19 infection, and rash.
- The overall incidence rate of TEAEs was 72%, with 35.7% determined to be related to ALTO-300 treatment.
- 5.0% of patients discontinued due to adverse events, and no material difference in the rate of TEAEs was observed between patients with or without the EEG biomarker.
In conclusion, the clinical data from the Phase 2a trial suggest that ALTO-300 has the potential to be effective as an adjunctive treatment for MDD, particularly in patients identified by a specific EEG biomarker profile. The treatment was generally safe and well tolerated.
For ALTO-300 in Major Depressive Disorder (MDD), potential approvable endpoints for regulatory agencies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) usually revolve around the safety and efficacy profile of the drug being tested. These endpoints are often derived from the symptoms' improvement scale scores and overall impact on patients' quality of life. Here are some commonly accepted potential endpoints:
- Efficacy Endpoints:
- Change in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score from baseline to a predefined time point.
- Response rate, usually defined as a ≥50% reduction in MADRS total score from baseline.
- Remission rate, defined as a MADRS total score below a certain low cut-off (such as ≤10).
- Health-related quality of life measures (e.g., the 36-Item Short Form Survey).
- Sustained response or remission rates, to assess the long-term benefits of ALTO-300.
- Safety Endpoints:
- Incidence of treatment-emergent adverse events (TEAEs).
- Serious adverse events (SAEs) and adverse events of special interest (AESIs).
- Changes in clinical laboratory values (e.g., LFTs), vital signs, physical findings, and other safety measures.
- Clinical Studies:
- Phase 3 randomized controlled trials (RCTs) are typically required to confirm the findings of Phase 2 trials and to collect more data on the drug's efficacy and safety. These trials will need to include a larger, more diverse patient population.
- Long-term extension studies to evaluate the safety and efficacy of ALTO-300 over an extended period.
- Patient Population:
- For Phase 3 trials, the FDA often recommends a larger and more varied demographic to enhance the generalizability of the results. This would involve several hundred to a few thousand patients per trial, depending on the statistical power needed to detect a clinically meaningful difference between the treatment and control groups.
Given that ALTO-300 is proposed as an adjunctive therapy for MDD, the trials might compare ALTO-300 in combination with a standard antidepressant versus placebo in combination with a standard antidepressant. This design better reflects the intended use of the drug in practice.
The number of patients required for these studies will depend on several factors, including the expected effect size, variability of response, acceptable alpha and beta error rates (Type I and II errors), and potential dropout rates. Drug developers often consult with statistical experts and may conduct a sample size calculation, considering the effect sizes seen in Phase 2 trials. For major depressive disorder, Phase 3 trials typically recruit anywhere from a few hundred to several thousand patients per treatment arm.
Considering ALTO-300 has shown efficacy in a patient population characterized by an EEG biomarker, confirmatory trials may focus on this subgroup, which could potentially lower the number of patients required if the effect size is large and consistent.
Ultimately, the design and size of the pivotal trials for ALTO-300 will be subject to discussions with regulatory authorities and will be influenced by the outcomes of earlier phase trials, current medical standards, the drug's mechanism of action, and the competitive landscape.
Financial model
Major Depressive Disorder
To create a hypothetical revenue build for ALTO-300 in Major Depressive Disorder (MDD), we will need to make several assumptions. Please note that all figures and calculations provided here are placeholders and hypothetical estimates, and should be adjusted based on real-world data as it becomes available. The process involves estimating the number of patients, treatment duration, pricing, market penetration, and adjustments for discounts and insurance coverage.
Here is a step-by-step build:
- Estimate the Target Population:
- Total population: Take the prevalence data for MDD in the market you are targeting (e.g., the United States).
- Patient segment: Estimate the percentage of MDD patients with an inadequate response to standard antidepressants, this will likely be a subset of the total MDD population.
- Calculate Treatable Population:
- Biomarker-specific group: Estimate the percentage of the inadequately responsive MDD patient segment with the EEG biomarker.
- Adjust for any other inclusion/exclusion criteria based on the Phase 2a study design.
- Estimate Market Penetration:
- Prescribing rates: Based on the drug's efficacy, safety, and competitive position in the market, estimate how quickly and widely ALTO-300 will be prescribed within the biomarker-specific population over a given timeframe (e.g., over the first year, second year, etc. of its launch).
- Determine Duration of Therapy:
- Average months per patient: Consider that the trial studied patients over eight weeks (about 2 months); evaluate the likelihood of continued treatment beyond this period.
- Estimate Treatment Cost:
- Drug pricing: Estimate pricing based on comparable adjunctive treatments for MDD and willingness to pay, possibly factoring in the novelty and efficacy of the biomarker identification.
- Gross-to-Net Adjustments: Anticipate discounts, rebates, and other adjustments to determine the net price. This may involve estimations of mandatory discounts (e.g., Medicaid), negotiated payer rebates, patient assistance programs, etc.
- Insurance Coverage:
- Coverage percentage: Estimate the percentage of patients whose insurance will cover ALTO-300.
- Patient out-of-pocket costs: A small percentage may be allocated to co-pays or co-insurance, affecting adherence and treatment continuation rates.
- Revenue Calculation:
Annual Revenue Estimate = (Treatable Population × Market Penetration × Insurance Coverage × (Average Duration of Therapy in Months/12) × Drug Price after Gross-to-Net Adjustments)
Let's build an example with hypothetical numbers:
- Target population segment = 10 million people (hypothetical number of inadequately responsive MDD patients)
- Biomarker-specific group = 40% (4 million people)
- Market penetration year 1 = 5%, year 2 = 10% (200,000 in year 1, 400,000 in year 2)
- Duration of therapy = 6 months on average
- Drug pricing = $500/month, Gross-to-Net Adjustments = 25% ($375 net)
- Insurance coverage = 90%
- Revenue year 1 = (4,000,000 × 5% × 90% × (6/12) × $375) = $337.5 million
- Revenue year 2 = (4,000,000 × 10% × 90% × (6/12) × $375) = $675 million
This model is extremely simplified and should include growth rates, patient discontinuation, competition effects, market expansion due to increased diagnostic rates or approval in additional indications, international market scaling, possible patent cliffs, and more. It's essential to update these estimates with real-world data, expert opinion, and additional market research as the information becomes available. Additionally, the use of data analytics and forecasting models can help refine revenue projections over time.
To estimate the probabilities of success at each stage of clinical development for ALTO-300 in Major Depressive Disorder (MDD), we can use the industry standard success rates for neurology products. However, it's important to note that these rates are general and can vary based on the therapeutic area, the specific drug profile, and other factors specific to the clinical development program. Here's how we would estimate the probabilities given the industry standard rates:
- Probability of Progression from Phase 2 to Phase 3 (P2 to P3):
- Industry Standard Probability for Neurology: 26.8%
- ALTO-300-specific factors: The Phase 2a data support the safety and efficacy of ALTO-300, potentially increasing the likelihood of progression relative to the industry standard. The identification of an EEG biomarker may also increase confidence in the target patient population.
- Estimated Probability of P2 to P3 Success: > 26.8% (Increase over industry standard based on positive Phase 2a results and biomarker identification; the exact increase would be speculative.)
- Probability of Phase 3 Success (P3 Success):
- Industry Standard Probability for Neurology: 53.1%
- ALTO-300-specific factors: Again, the promising Phase 2a results could suggest a higher probability of success; however, Phase 3 trials are larger and more definitive. Note that many drugs fail in Phase 3 due to lack of efficacy or unseen safety issues in a larger patient population.
- Estimated Probability of P3 Success: > 53.1% (increase is assumed based on the robustness of Phase 2a data and the presence of the EEG biomarker; actual increment would be subject to further statistical and expert evaluation.)
- Probability of FDA Submission Success (FDA Success):
- Industry Standard Probability for Neurology: 86.7%
- ALTO-300-specific factors: If a drug reaches the submission stage, it means that it has already achieved positive results in Phase 3 trials. ALTO-300's strong safety profile and EEG biomarker specificity could be favorable for regulatory approval. However, regulatory success also heavily depends on the robustness of clinical data and risk-benefit assessment.
- Estimated Probability of FDA Success: > 86.7% (Increase assumes that strong Phase 3 data aligned with Phase 2 outcomes, in combination with an unmet need in MDD, could drive a favorable review; however, regulatory risks always apply.)
These probabilities are multiplicative when considering the overall likelihood of approval from the current stage. Therefore, the overall estimated probability of ALTO-300's success from the end of Phase 2 to approval would be the product of each stage's success rate:
ALTO-101
The therapeutic rationale for using a PDE4 inhibitor like ALTO-101 in Cognitive Impairment Associated with Schizophrenia (CIAS) is rooted in the crucial role of the cyclic adenosine monophosphate (cAMP) signaling pathway in cognitive functions and neuroplasticity.
CIAS is a challenging aspect of schizophrenia characterized by deficits in memory, attention, executive function, and various other cognitive domains, which significantly impact patient quality of life and functional outcomes. Current treatments for schizophrenia predominantly target the positive symptoms (hallucinations, delusions), with a notable gap in efficacious treatments for cognitive and negative symptoms.
Phosphodiesterase 4 (PDE4) is an enzyme that degrades cAMP, a ubiquitous second messenger involved in a range of cellular functions, including neuronal signaling. The cAMP pathway is known to have a significant role in the modulation of immune and inflammatory responses, learning, memory, and mood regulation. In patients with schizophrenia and other cognitive disorders, there has been a documented reduction in neuroplasticity-related signaling pathways, including the cAMP pathway. This dysregulation is thought to contribute to the cognitive deficits observed in such conditions.
As a PDE4 inhibitor, ALTO-101 aims to prevent the breakdown of cAMP, thereby increasing its levels in the brain. Elevating cAMP levels is believed to enhance neuroplasticity, which is vital for learning and memory processes. This is especially relevant in the hippocampus, a brain region integral to memory formation and cognitive function. In preclinical models, increasing hippocampal cAMP has been shown to improve various forms of memory and provide pro-cognitive effects.
Early phase studies of ALTO-101 have demonstrated that it can enter the human brain and is well-tolerated. Additionally, preliminary results suggest that ALTO-101 may have robust effects on cognitive processing and performance as measured by EEG and cognitive tests. These promising findings provide a strong basis for advancing the clinical development of ALTO-101 into Phase 2 proof-of-concept trials in patients with CIAS.
In summary, the use of ALTO-101 in CIAS capitalizes on the understanding that enhancing cAMP signaling through PDE4 inhibition may correct underlying neurochemical deficits associated with cognitive impairments in schizophrenia, potentially providing a novel therapeutic avenue to address these unmet clinical needs.
The science underlying the use of PDE4 inhibitors for cognitive enhancement is based on a wealth of preclinical evidence; however, its translation into clinical practice is still an area of active research and development.
The understanding that cAMP signaling pathways play a significant role in cognitive functions and neuroplasticity is well established. cAMP is a critical second messenger in numerous biological processes, and its role in the brain includes the regulation of gene transcription, neuronal excitability, synaptic plasticity, and memory formation. Specifically, the involvement of cAMP signaling in learning and memory has been documented across various types of animal models.
Furthermore, post-mortem and genetic studies indicating alterations in cAMP signaling in individuals with cognitive disorders provide a compelling rationale for targeting this pathway to improve cognitive deficits. PDE4, being one of the principal enzymes regulating cAMP levels by catalyzing its breakdown, emerges as a plausible target for pharmacological intervention.
However, here are some aspects that are subject to uncertainty or debate:
- Translating preclinical findings to humans: Animal models do not perfectly replicate human cognitive impairment or schizophrenia. Hence, findings in preclinical studies might not always predict clinical success in humans.
- PDE4 inhibitor specificity: PDE4 is a broad class with several subtypes (PDE4A, 4B, 4C, 4D), and the distribution of these subtypes can vary in different tissues. It remains to be seen whether ALTO-101 has selective inhibition profiles that could lead to fewer side effects and improved efficacy.
- Therapeutic window and side effects: Historically, PDE4 inhibitors have shown a narrow therapeutic window and dose-limiting side effects, such as nausea and emesis, largely related to PDE4 inhibition in peripheral tissues. This poses a challenge for achieving brain concentrations that are both effective and tolerable.
- Complex nature of schizophrenia: Schizophrenia is highly heterogeneous, and cognitive impairments arise from a complex interplay of genetic, neurobiological, and environmental factors. It remains unclear how a single therapeutic approach targeting the cAMP pathway will affect the broad range of symptoms.
- Long-term effects: The long-term consequences of sustained PDE4 inhibition and increased cAMP signaling in the brain are not well understood.
The overall level of evidence suggests that the theoretical basis for using PDE4 inhibitors such as ALTO-101 for cognitive enhancement is strong due to a well-documented role of the cAMP pathway in cognition and neuroplasticity. The early clinical data on safety and brain penetration of ALTO-101 add to the support. However, the clinical efficacy, particularly in Phase 2 and 3 trials, will be the ultimate test of the validity of this therapeutic approach.
The leap from proof of concept in early-phase trials to broader clinical application will require addressing the above uncertainties and will depend on a balance between efficacy, safety, and tolerability. Only with the completion of more extensive trials can the place of PDE4 inhibitors in the treatment of CIAS be fully determined.
Research into the PDE4 enzyme's role in cognitive impairment, including its association with schizophrenia, is supported by numerous preclinical studies and some clinical investigations, though it remains an emerging field. Here's a synthesis of key points from the literature:
- PDE4 Subtypes in the Brain: PDE4 has several subtypes (PDE4A, 4B, 4C, 4D), which are differentially expressed in brain regions critical for cognitive function, such as the prefrontal cortex and hippocampus. These subtypes are thought to play distinct roles in neuronal signaling and synaptic plasticity, which are processes disrupted in schizophrenia (Zhang HT. PDE4 as a target for cognition enhancement. Expert Opin Ther Targets. 2009;13(6):707-18).
- Animal Studies: Many studies using PDE4 inhibitors in rodents have suggested improved cognitive performance in tasks designed to assess learning, memory, and executive function. For example, PDE4 inhibition enhanced long-term potentiation (LTP), a cellular mechanism for learning and memory, suggesting potential benefits for cognitive deficits (Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl). 2009;202(1-3):419-43).
- Genetic and Pathophysiology Evidence in Schizophrenia: Genetic associations have indicated that variations in PDE4B are linked to susceptibility to schizophrenia. Moreover, deficiencies in cAMP signaling pathways, which would be modulated by PDE4 activity, have been implicated in the cognitive deficits of schizophrenia (Numata S, Iga JI, Nakataki M, Tayoshi S, Tanahashi T, Itakura M, Ueno SI, Ohmori T. Positive association of the PDE4B gene with schizophrenia in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):375-9).
- CNP Disorders and PDE4: Neuroinflammation and altered intracellular signaling pathways are common features in various central nervous system (CNS) disorders, including schizophrenia. PDE4 inhibitors have been shown to have anti-inflammatory effects and are proposed to address these common pathophysiological traits (Giordano D, Magalon K, Perreau VM, Durbec P, Roman FS, Krebs MO, Poirier MF, Abrous DN, Barneoud P, Alescio-Lautier B. Impact of PDE4D deficiency on olfactory learning and memory in mice. Learn Mem. 2012;19(5):195-9).
- Human Studies: While extensive human studies are lacking, there is some evidence from early-phase trials in conditions such as Alzheimer's disease suggesting that PDE4 inhibitors may have potential cognitive benefits. However, these findings are preliminary and further clinical evaluation is necessary to establish efficacy in schizophrenia specifically (Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15(14):1688-98).
- Clinical Trials: Initial clinical trials designed to study the effect of PDE4 inhibitors on cognitive impairment have shown promise, but many have been limited by side effects, as previously mentioned. The specificity of ALTO-101 for brain PDE4 subtypes may offer a potential advantage in mitigating these side effects.
In summary, while there is compelling evidence from preclinical studies indicating that PDE4 plays a role in cognition and could be a therapeutic target for cognitive impairment, clinical validation of PDE4 inhibitors for the treatment of CIAS is still in progress. The ongoing and future clinical trials will be paramount in confirming whether PDE4 inhibition can translate into a clinically meaningful improvement in cognitive impairments associated with schizophrenia.
The therapeutic rationale for targeting PDE4 in Cognitive Impairment Associated with Schizophrenia (CIAS) is built on a mixture of mechanistic insights, preclinical data, and early clinical results. Here is an evaluation of the strengths and weaknesses of this evidence base:
Strengths:
- Mechanistic Plausibility: The cAMP signaling pathway is critically involved in fundamental neural processes such as long-term potentiation (LTP), neuroplasticity, and neuroinflammation, which underpin learning and memory functions. PDE4's role in degrading cAMP directly links it to the modulation of this pathway.
- Preclinical Data: A vast array of animal studies supports the idea that PDE4 inhibitors can enhance cognitive functions. Animal models have shown that increasing cAMP via PDE4 inhibition leads to improved performance in a variety of tasks that measure memory and cognition.
- Genetic Associations: Genetic studies in humans have found associations between PDE4B gene variants and schizophrenia, which supports the relevance of PDE4 to the disease.
- Initial Clinical Safety: Early clinical studies of ALTO-101 have indicated it is well-tolerated and can penetrate the human brain, which is essential for any CNS-active drug.
- Neurobiological Diversity and Targeting: PDE4 has multiple subtypes with distinct expression patterns in the brain. This offers the possibility of creating subtype-selective inhibitors that minimize side effects and maximize therapeutic effects.
- Unmet Medical Need: Since there are no approved treatments for CIAS, if PDE4 inhibitors prove effective, they would address a significant gap in care for patients with schizophrenia.
Weaknesses:
- Translational Hurdles: Animal models do not fully represent the complexities of human psychiatric conditions. Consequently, treatments that are effective in animal models do not always work in humans.
- Side Effect Profile: PDE4 inhibitors have historically been associated with gastrointestinal side effects that limit their use. If this holds true for ALTO-101, it could be a significant barrier to adherence and widespread use.
- Complex Etiology of Schizophrenia: CIAS likely derives from a multiplicity of interacting genetic and environmental factors, which might not be fully addressed through a single pharmacological target such as PDE4.
- Subtype Specificity: It's unclear whether ALTO-101 is sufficiently selective for certain PDE4 subtypes to optimize cognitive enhancement while minimizing side effects. Better selectivity could mean increased effectiveness and more favorable safety profiles, but finding the right balance is a substantial challenge.
- Long-Term Effects Unknown: Long-term cognitive and psychiatric effects of chronic PDE4 inhibition are not yet understood, and drug tolerance could potentially limit long-term use.
- Clinical Evidence Base: Although preliminary clinical studies have been encouraging, the evidence for PDE4 inhibitors in the treatment of CIAS is not yet established in Phase 2 or Phase 3 trials. The efficacy, optimal dosing, and treatment duration are still unclear.
- Sustainability of Treatment Benefits: Even if PDE4 inhibitors like ALTO-101 show initial cognitive benefits, maintaining these improvements over time and demonstrating functional outcomes are critical unanswered questions.
In conclusion, while the rationale for using a PDE4 inhibitor to treat CIAS is underpinned by a strong scientific hypothesis and promising early trial data, there remain substantial gaps that need to be addressed through rigorous clinical research. The path from bench to bedside is particularly fraught in psychiatric drug development, and the substantial evidence base from preclinical models will need strong clinical trial results to be considered robust.
Clinical trial overview
The clinical data for ALTO-101 in the context of cognitive impairment associated with schizophrenia (CIAS) is derived from several Phase 1 trial outcomes as follows:
- Tolerability: ALTO-101 was well tolerated in 154 healthy subjects and 11 Parkinson’s disease patients at a range of doses (0.05 mg to 4.5 mg). The compound is observed to have an acceptable tolerability profile.
- Blood-Brain Barrier Penetration: In a positron emission tomography (PET) study, ALTO-101 successfully crossed the blood-brain barrier, which is crucial for its potential effects on brain-related disorders.
- Dose Selection: Data from these studies were used for dose selection based on target engagement and tolerability for further clinical development in CIAS.
- Pharmacodynamics and Neurocognitive Effects: In a cross-over design study with 40 healthy adults, single doses of ALTO-101 at 0.5mg and 1.5mg demonstrated significant pro-cognitive pharmacodynamic effects, showing:
- Reduced resting theta power in EEG (often elevated in psychiatric disorders).
- Increased gamma-band phase locking (commonly reduced in schizophrenia).
- Enhanced mismatch negativity (blunted in schizophrenia).
- Cognitive Performance: Significant, dose-dependent improvements were observed in information processing speed and on a global cognition composite measure.
- Side Effects: A dose-dependent increase in nausea, dizziness, and lightheadedness was observed, with higher incidence at the 1.5mg dose. These were typically temporally linked to the time to reach peak drug concentration (Tmax). Only a small percentage of patients discontinued due to adverse events, and no serious treatment-emergent adverse events (TEAEs) were identified.
- Transdermal Reformulation: To mitigate the side effects associated with peak brain concentrations of oral dosing, ALTO-101 has been reformulated for transdermal delivery, which is expected to provide a more consistent dose and potentially reduce nausea-related adverse events.
In summary, the Phase 1 clinical data provides evidence that ALTO-101 may have pro-cognitive effects in CIAS and is generally well-tolerated. The data also led to improvements in drug delivery, with the development of a transdermal formulation to enhance tolerability and treatment experience for patients.
Applicable endpoints for clinical trials in Cognitive Impairment Associated with Schizophrenia (CIAS) typically focus on cognitive function, safety, tolerability, and overall clinical effectiveness of the interventional drug. The U.S. Food and Drug Administration (FDA) provides guidance on endpoints and study design for drugs targeting CIAS. The following are possible approvable endpoints and outlines of clinical studies for ALTO-101 development:
- Cognitive Testing:
- Primary Endpoints: Use of standardized and validated neurocognitive test batteries such as the MATRICS Consensus Cognitive Battery (MCCB), which covers domains of cognition that are usually impaired in schizophrenia. Improvements in scores, particularly in processing speed, attention, working memory, verbal learning, and executive function, may serve as primary endpoints.
- Secondary Endpoints: Changes in functional capacity assessments, clinician-based scales of cognitive function such as the CGI-Cognition scale, or patient-reported outcome measures.
- Biomarker Analysis:
- Biomarkers derived from EEG or event-related potentials may be incorporated as exploratory endpoints to provide physiological evidence of drug activity.
- Safety and Tolerability:
- Standard measures include the incidence of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), laboratory tests, vital signs, and physical examinations. A particular emphasis may be placed on monitoring the frequency and severity of gastrointestinal symptoms, as well as dizziness and lightheadedness that were identified in Phase 1 studies.
- Clinical Trials:
- Phase 2 Trials: These are proof-of-concept studies typically designed to obtain preliminary data on the effectiveness of ALTO-101, to determine the appropriate dose for phase 3 trials, and to continue to evaluate safety. Sample size here may vary from tens to a few hundred patients.
- Phase 3 Trials: These trials confirm the therapeutic benefits observed in Phase 2, assess the overall risk-benefit profile, and provide more rigorous data on safety. Phase 3 trials often require larger sample sizes, commonly ranging from several hundred to thousands of patients, depending on the variability and expected effect size.
- Trial Duration:
- Treatment duration in Phase 2 trials may be shorter, ranging from weeks to a few months. For Phase 3, trial duration may extend to 6 months or longer to thoroughly assess cognitive improvements and to monitor for long-term safety.
- Study Population:
- The patient population for both phases will include individuals diagnosed with schizophrenia and objectively measured cognitive deficits. This may include drug-naïve patients, those on stable antipsychotic regimens, or subgroups based on genetic, cognitive, or symptom profiles.
- Estimated Patient Numbers:
- A Phase 2 study might range from 50-300 subjects, but this could vary widely based on the study design and the specific endpoints.
- Phase 3 studies, which are larger and more definitive, could require anywhere from 300 to over 1000 patients for sufficient statistical power to detect clinically significant changes.
In summary, a typical clinical development plan for ALTO-101 in CIAS would include Phase 2 and Phase 3 studies, assessing both cognitive outcomes using standard batteries and safety/tolerability measures, with the latter studies enrolling hundreds to thousands of patients. The precise design of these studies, including choice of endpoints and determination of sample size, would likely evolve in consultation with regulatory agencies and as more data are collected from earlier-phase trials.
Market overview
Cognitive impairment is a core feature of schizophrenia, a chronic and severe mental disorder characterized by a range of symptoms that can affect thinking, behavior, emotions, and perception of reality. Cognitive deficits often emerge before the onset of hallmark psychotic symptoms such as hallucinations and delusions and are among the most disabling features of the illness, impacting daily functioning and quality of life.
While the exact pathophysiology of cognitive impairment in schizophrenia is not fully understood, it is believed to involve dysregulation of various neurotransmitter systems, notably dopamine, glutamate, and GABA. There is also evidence of structural brain changes, including reductions in gray matter volume, particularly in the frontal and temporal cortices, and deficits in white matter integrity. These alterations are thought to lead to disruptions in neural circuitry that underlie cognitive processes.
Cognitive deficits in schizophrenia encompass a broad range of domains, including:
- Attention and Concentration: Difficulty in sustaining focus on tasks and being easily distracted.
- Memory: Impairments in both working memory (the ability to hold and manipulate information in short-term) and long-term memory, including difficulties with learning new information.
- Executive Function: Problems with planning, problem-solving, and engaging in goal-directed behaviors.
- Processing Speed: Slowed mental processing that can affect various aspects of cognition and daily functioning.
- Verbal Fluency and Language: Challenges with finding words, speaking coherently, or understanding complex language.
- Social Cognition: Difficulties in recognizing social cues, managing emotions, and engaging effectively in social situations.
These cognitive deficits can occur independently of the psychotic symptoms and often persist even when other symptoms are managed with medication.
Cognitive impairment in schizophrenia tends to be stable over time, and in some cases, it may worsen with the progression of the disease. These deficits are typically less responsive to antipsychotic medications than the psychotic symptoms. As a result, they are a major determinant of long-term disability and a critical target for interventions aimed at improving overall prognosis.
The management of cognitive impairment in schizophrenia includes pharmacological and non-pharmacological approaches:
- Pharmacotherapy: While current antipsychotics primarily target positive symptoms, there is ongoing research into medications that can specifically improve cognitive deficits. Treatments that target neurotransmitter systems such as glutamate (e.g., via NMDA receptor modulators) are being explored.
- Cognitive Remediation: This therapy involves structured tasks designed to improve cognitive function. It can be computer-based or involve direct training and practice in cognitive tasks.
- Cognitive Behavioral Therapy (CBT): While CBT is primarily used to treat the negative and positive symptoms of schizophrenia, it can also be adapted to help patients develop strategies to cope with cognitive impairments.
- Psychosocial Interventions: Supported employment, social skills training, and other psychosocial interventions can help individuals with schizophrenia manage the impact of cognitive deficits on their daily lives.
Research continues to search for more effective treatments for cognitive deficits in schizophrenia. Investigations range from pharmacological approaches including novel compounds, combination therapies, and personalized medicine strategies based on genetic profiles, to advanced neurostimulation techniques like transcranial magnetic stimulation (TMS).
In summary, cognitive impairment in schizophrenia is a critical determinant of functional outcomes and remains a major area of unmet need. Understanding and addressing these deficits is key to improving the prognosis and enhancing the quality of life for individuals living with schizophrenia.
To provide an analysis of the market opportunity for a drug like ALTO-101 for Cognitive Impairment Associated with Schizophrenia (CIAS), it is essential to examine the competitive landscape, current standard of care, and unmet medical needs within this indication.
There are currently no drugs approved by the U.S. Food and Drug Administration (FDA) specifically for CIAS, though some currently approved antipsychotic medications may have mild cognitive benefits for patients. Medications such as donepezil (a cholinesterase inhibitor used for Alzheimer's disease) have been studied in schizophrenia with mixed results. Hence, the competition for a new effective drug in this domain would likely be sparse, presenting a prime opportunity if ALTO-101 shows significant efficacy.
The current standard of care for schizophrenia includes atypical antipsychotics, with adjunctive treatment options like cognitive behavioral therapy and cognitive remediation therapy for cognitive deficits. However, these do not fully address the cognitive impairments, thus leaving a significant gap in treatment options.
There is an unmet medical need due to:
- Existing therapies primarily address positive symptoms, with limited efficacy on cognitive deficits.
- Persistent cognitive deficits in schizophrenia patients are a major cause of disability and decreased quality of life.
- Current non-pharmacological interventions require significant resources and are not universally available or effective for all patients.
The global antipsychotic drug market is a multi-billion dollar industry, and a targeted cognitive-enhancing drug like ALTO-101 would likely address a sizeable subset of this market. Considering the prevalence of schizophrenia (approximately 1% of the population worldwide) and the fact that most individuals with the disorder exhibit cognitive deficits, the target market for an effective CIAS treatment would be extensive.
There are several companies working on developing treatments for cognitive impairment associated with schizophrenia (CIAS) that may compete with ALTO-101. These organizations range from large pharmaceutical companies to smaller biotech firms and are investigating various pharmacological approaches.
Financial model
Generating a hypothetical revenue build for ALTO-101 in Cognitive Impairment Associated with Schizophrenia (CIAS) involves making various assumptions and providing placeholder estimates for the number of patients treated, pricing, insurance coverage, etc. Below is a revenue build model with sample assumptions and calculations:
- Target Population Size:
- Prevalence of Schizophrenia: Assume 1.5 million diagnosed in the U.S.
- Percentage with CIAS: 80% of schizophrenia patients have CIAS = 1.2 million patients.
- Treatment Market Penetration:
- Assume ALTO-101 captures 25% of the CIAS market
- Insurance Coverage and Access:
- Percentage of Insured Patients: 90% are covered by some form of insurance.
- Market Access Success Rate: 70% success rate in achieving formulary coverage by major insurance providers.
- Pricing:
- Assume an annual cost of therapy for ALTO-101 is $30,000 (based on the complexity and severity of the disease, cost of similar therapies, and willingness to pay).
- Gross-to-Net Adjustments:
- Include discounts, rebates, and other price concessions typical in the pharmaceutical industry. Assume a 30% gross-to-net discount = $30,000 * (1 - 0.30).
- Duration of Therapy:
- Assume average duration of therapy per patient is 1 year (considering chronic administration with potential discontinuations).
- Probability of Progression from Phase 1 to Phase 2: 47.7%
- Probability of Phase 2 Success: 26.8%
- Probability of Phase 3 Success: 53.1%
- Probability of FDA Submission Success: 86.7%
- Dopamine and Anhedonia: Anhedonia in MDD is thought to be related to dysfunction in dopaminergic signaling in the brain's reward system, particularly within the nucleus accumbens. Enhancing dopamine release in this area could improve symptoms of anhedonia and, consequently, other related depressive symptoms.
- Histamine H3 Receptor Role: The H3 receptor has an auto-regulatory role in the brain and can inhibit the release of several neurotransmitters, including dopamine. Thus, by functioning as an inverse agonist to the H3 receptor, ALTO-203 is designed to upregulate dopaminergic neurotransmission by removing the inhibitory effect on dopamine release.
- ALTO-203 Differentiation: Unlike other H3 receptor inverse agonists, such as pitolisant, which has not shown effects on dopamine release in the nucleus accumbens, ALTO-203 has demonstrated in preclinical studies the ability to elevate dopamine levels in this specific brain region. This suggests that it might more effectively address the dopaminergic dysfunction believed to be associated with anhedonia in MDD.
- Comparison with Modafinil: In Phase 1 trials, ALTO-203 showed an acute increase in positive emotions to levels equivalent to or greater than modafinil, which acts through dopamine release and is approved for use in conditions such as narcolepsy. Modafinil has also shown efficacy in treating depressive symptoms in MDD and bipolar depression, providing indirect evidence that increasing dopamine can have antidepressant effects.
- Potential Cognition Enhancements: By increasing dopamine, particularly in the reward system, ALTO-203 may also indirectly enhance cognitive and motivational processes, which are often impaired in patients with MDD and anhedonia.
- Patent Protection: With a robust intellectual property estate extending protection up to at least 2044, ALTO-203 presents a long-term potential as a therapeutic intervention for MDD if it proves to be effective and safe in clinical trials.
- Dopaminergic Dysfunction and Anhedonia: The link between dopaminergic dysfunction in the brain's reward circuitry, particularly in the nucleus accumbens, and symptoms of anhedonia in MDD is well-established. Dopamine plays a key role in reward signaling and motivation, and deficits in this system are thought to contribute to anhedonia.
- H3 Receptor Role: The function of H3 receptors as autoreceptors and heteroreceptors that modulate the release of various neurotransmitters, including dopamine, is well understood. Inhibiting these receptors has the potential to increase neurotransmitter levels, which has been explored in various contexts such as sleep-wake disorders and cognitive impairment.
- Pharmacological Activity of Modafinil: The efficacy of modafinil in enhancing alertness, particularly through its effects on dopamine, has been demonstrated in the context of narcolepsy and other sleep disorders. There is also evidence of its off-label use in MDD and bipolar depression to address fatigue and improve mood.
- Specific Mechanism of H3 Inverse Agonists: While it is known that H3 inverse agonists can increase neurotransmitter release, the exact mechanism by which this occurs, especially in relation to mood disorders, is complex and not fully elucidated. The brain is highly interconnected, and neurotransmitter systems interact in intricate ways that are not completely understood.
- Efficacy of H3 Inverse Agonists in MDD with Anhedonia: The effectiveness of H3 inverse agonists specifically for treating anhedonia in MDD is still under investigation. Clinical trial results for ALTO-203 and others in this class are needed to establish their efficacy and safety profiles for this indication.
- Translation from Preclinical to Clinical Outcomes: The translation of positive effects in animal models (such as increases in dopamine release in the nucleus accumbens) to clinical outcomes in humans is always uncertain. Animal models can be informative, but they do not always predict clinical efficacy in humans due to differences in brain complexity, receptor distributions, and the multifactorial nature of human mood disorders.
- Preclinical Studies: Rodent studies provide the foundation for understanding the H3 receptor's involvement in mood regulation. H3 receptor antagonists and inverse agonists have shown antidepressant-like effects in animal models of depression, supporting the hypothesis that these compounds could modulate neurotransmission in pathways related to mood disorders.
- Neurotransmitter Modulation: H3 receptors, when blocked or inversely agonized, can enhance the release of dopamine and other neurotransmitters in brain regions implicated in reward processing circuitry, such as the nucleus accumbens. This neurochemical change is theorized to potentially counteract the dopaminergic deficits that are associated with anhedonia in MDD.
- Clinical Trials with Other H3 Antagonists/Inverse Agonists: There are scarce clinical trials directly investigating H3 receptor antagonists or inverse agonists for the treatment of anhedonia in MDD. However, the wake-promoting agent pitolisant, an approved H3 receptor antagonist/inverse agonist for narcolepsy, has hinted at potential cognitive and mood benefits, though its primary indications are not for depression.
- Pharmacological Plausibility: There is a sound pharmacological foundation for using H3 receptor inverse agonists to potentially enhance neurotransmitter release, particularly dopamine, which is linked to the reward pathway and anhedonia.
- Preclinical Data: Rodent studies have shown that H3 receptor inverse agonists can increase neurotransmitter release in the brain's reward system, which provides a biological basis for their potential antidepressant effects.
- Modafinil as an Indirect Evidence: Clinical success of modafinil, a drug that increases dopamine levels and has shown benefits in mood and cognitive symptoms in MDD and bipolar depression, indirectly supports the rationale that enhancing dopaminergic transmission could be beneficial for anhedonia.
- Neurobiological Targeting: The approach of targeting a specific neurobiological mechanism involved in the symptoms of MDD (i.e., a reduction in dopamine signaling in the nucleus accumbens) is a strength because it offers a more tailored treatment strategy.
- Translation from Animal Models to Humans: There is often a significant gap between results obtained in animal models and those obtained in human clinical trials. The complexity of human MDD and anhedonia may not be fully replicated in preclinical studies.
- Limited Clinical Data: As of early 2023, there is a paucity of clinical trial data specifically evaluating the efficacy of H3 receptor inverse agonists for anhedonia in MDD. Thus, the therapeutic potential remains to be definitively proven in this patient population.
- Complexity of MDD and Anhedonia: MDD is a multifaceted disorder with a diversity of symptoms and pathophysiological factors. Anhedonia itself is complex and might not be solely attributable to dopaminergic dysfunction, raising the question of whether enhancing dopamine alone can address all related aspects of the symptomatology.
- Specificity and Selectivity: While blocking H3 receptors can theoretically enhance dopamine release, it also affects the release of other neurotransmitters. The broad action could lead to unintended side effects, calling for selectivity in drug targeting.
- Variability in Patient Response: There is considerable inter-individual variability in the response to antidepressants, and it is unclear how different subgroups of patients with MDD will respond to H3 receptor inverse agonists. The role of genetic, environmental, and other biological factors in modulating treatment response is still not fully understood.
Safety and Tolerability: ALTO-203 was evaluated in three Phase 1 clinical studies involving healthy subjects. The originator of ALTO-203 conducted these studies between 2009 and 2014, which were aimed at assessing safety and pharmacokinetics. In these trials, ALTO-203 was well tolerated by the participants. The first study tested single doses ranging from 0.02 mg up to 5.0 mg in 48 healthy subjects. The second study further assessed safety and dosing, including single and multiple doses from 0.02 mg up to 0.5 mg over nine days in 48 healthy subjects.
Predictable Pharmacokinetics: Across the Phase 1 trials, ALTO-203 exhibited predictable pharmacokinetics, which is critical for determining appropriate dosing regimens.
Efficacy Indicators: In one of the trials that used a cross-over design with 40 healthy individuals, ALTO-203 at a single dose of 25µg showed a positive effect on subjective emotion. This was measured using the Bond and Lader scale components that evaluate alertness and mood, indicating that ALTO-203 may have a beneficial impact on emotional states.
Comparison to Controls: The same trial included two active control arms, modafinil (a dopaminergic agent) and donepezil (a cholinergic agent), which allowed for comparison of the effects of ALTO-203. The placebo-adjusted differences in effects on mood and alertness were similar to or greater than those achieved by modafinil, whereas donepezil did not show a significant deviation from placebo.
Additional Cognitive Findings: Besides subjective emotional improvements, additional data from the cross-over trial indicated that ALTO-203 improved reaction time and adaptive eye tracking in participants, suggesting potential cognitive benefits.
Informed Development Plan: The current developer has used the data from the Phase 1 trials to inform the clinical development plan for ALTO-203, particularly focusing on dosing, tolerability, and acute pharmacodynamic effects as they pursue treatment options for Major Depressive Disorder with anhedonia.
- Hamilton Depression Rating Scale (HDRS): Specifically, the HDRS-17 or HDRS-24 with attention to anhedonia-related items. These scales are frequently used in clinical trials for MDD.
- Montgomery-Åsberg Depression Rating Scale (MADRS): This is another common scale where items related to pleasure and interest can be used to assess anhedonia.
- Snaith-Hamilton Pleasure Scale (SHAPS) or Chapman Scales for Anhedonia: These scales are specifically designed to measure hedonic tone and could provide specific insights into the treatment effects on anhedonia.
- Clinical Global Impressions (CGI): This is a subjective but widely used measure that can assess overall improvement and severity of illness.
- Phase 2 Trials: These are proof-of-concept studies designed to explore the efficacy of ALTO-203 in a MDD population, including a focus on anhedonia. A randomized, double-blind, placebo-controlled design is standard. Sample sizes can vary greatly but typically range from a few dozen to a couple of hundred patients. This phase would involve multiple doses to find the optimal dose that balances efficacy and side effects.
- Phase 3 Trials: If Phase 2 trials demonstrate positive results, Phase 3 trials are larger studies that confirm the efficacy, safety, and tolerability of ALTO-203. These studies are pivotal for FDA approval and would likely involve several hundred to a few thousand patients. They often include comparator arms with existing treatments.
- Long-term Safety Studies: These studies track participants for extended periods to monitor for any long-term side effects or issues that may arise from prolonged use of the medication.
- Total population: Estimate the total number of individuals with MDD in the market region (e.g., the United States).
- Prevalence of Anhedonia in MDD: Estimate the proportion of MDD patients experiencing anhedonia to find the eligible treatment pool.
- Treatment-seeking rate: Project the percentage of individuals with MDD and anhedonia likely to seek treatment (considering stigma, awareness, health care access, etc.).
- Diagnosis rate: Estimate the percentage of treatment seekers who are properly diagnosed and classified for potential treatment with ALTO-203.
- Market penetration: Estimate the adoption rate of ALTO-203 by healthcare providers as a valid treatment option.
- Treatment duration: Estimate the average treatment duration per patient (e.g., 6 months, 1 year).
- Patient adherence rate: Consider adherence to medication, estimating the percentage of patients who will complete the prescribed treatment course.
- Gross revenue: Calculate potential gross revenue by multiplying the eligible patient population by the price and treatment duration.
- Gross-to-net adjustments: Deduct estimates for discounts, rebates, patient assistance programs, etc., to project the net revenue.
- Insurance coverage: Estimate the percentage of treatment costs covered by insurance payers, and the impact on pricing and patient uptake.
- Market growth rate: Adjust revenue projections annually for market growth/decline, new competition, etc.
- Calculate the number of patients treated annually (eligible patient population * market penetration * treatment-seeking rate * diagnosis rate).
- Estimate the total annual treatment duration for all patients (number of patients treated * average treatment duration).
- Estimate total annual gross revenue (total annual treatment duration * price * adherence rate).
- Estimate total annual net revenue by applying gross-to-net adjustments and considering insurance coverage impacts.
- Total population with MDD in the U.S.: 17 million
- Prevalence of Anhedonia in MDD: 30%
- Treatment-seeking rate: 50%
- Diagnosis rate: 80%
- Market penetration: 5% Year 1, growing to 15% Year 5
- Eligible patient population: 17M * 30% * 50% * 80% = 2.04M (Year 1)
- Pricing: $500/month
- Treatment duration: 6 months average
- Patient adherence rate: 75%
- Gross-to-net adjustments: 20% off the gross revenue
- Glutamatergic System Dysregulation in MDD: Research has increasingly pointed to the role of the glutamatergic system in mood regulation and the pathophysiology of depression. Glutamate is the primary excitatory neurotransmitter in the brain, and its transmission occurs mainly through NMDA (N-methyl-D-aspartate) receptors. Abnormalities in glutamate neurotransmission are linked with MDD, and studies have revealed altered levels of glutamate in various brain regions of depressed individuals.
- Role of NMDA Receptors: NMDA receptors are ion channels that modulate synaptic plasticity, which is crucial for learning, memory, and neural network flexibility. These receptors are composed of several subunits, including GluN2B. Hyperfunction of NMDA receptors can lead to excitotoxicity and neuronal damage, which has been implicated in the onset of depressive symptoms.
- Antidepressant Effects of NMDA Antagonists: Compounds that antagonize NMDA receptors, most notably ketamine and its enantiomer esketamine, have been shown to produce rapid antidepressant effects even in treatment-resistant depression. This has been attributed to their ability to modulate synaptic plasticity rapidly, increase the release of neurotrophic factors, and induce synaptogenesis.
- Targeting GluN2B Subunit: GluN2B selective antagonism may offer advantages over nonselective NMDA receptor blockade. By specifically targeting the GluN2B subunit, it's possible to modulate the receptor's function without completely inhibiting it, thereby potentially reducing the risk of side effects associated with broader spectrum NMDA receptor antagonists.
- Potential for Rapid Onset: ALTO-202 has not shown statistically significant changes compared to placebo in the primary endpoint during a previous trial. However, secondary analysis suggested potentially clinically meaningful rapid-onset antidepressant effects. This hints at the possibility that ALTO-202 could alleviate depressive symptoms more quickly than traditional antidepressants, which can take weeks to show full therapeutic effects.
- Safety Profile: The tolerability of ALTO-202 in prior clinical trials suggests that it might be a safe option for patients. With the most common adverse events being increased blood pressure, dizziness, somnolence, and paresthesia, and no serious treatment-related adverse events, it presents a potentially safer profile compared to other NMDA antagonists.
- Glutamatergic System Dysregulation in MDD: The involvement of the glutamatergic system in MDD has been supported by numerous studies, with elevated glutamate levels observed in certain areas of the brains of individuals with depression. However, the exact nature of this dysregulation and its causative role in the etiology of MDD is still not fully understood.
- Efficacy and Mechanism of NMDA Antagonists: The rapid antidepressant effects of NMDA receptor antagonists like ketamine are well-documented through both clinical trials and real-world use. The exact mechanisms, however, are still being elucidated. While it's clear that they modulate synaptic plasticity and stimulate neurotrophic pathways, the most critical pathways for their antidepressant effects are not definitively established.
- Specificity of GluN2B Antagonists: The concept of selective antagonism of GluN2B-containing NMDA receptors is based on the hypothesis that it could reduce side effects compared to non-selective antagonists. Nevertheless, the clinical benefits and safety profile of selective GluN2B antagonism as a superior alternative to broader NMDA receptor antagonism are still to be conclusively proven in large-scale clinical trials.
- Rapid Onset of Antidepressant Effects: ALTO-202 and similar drugs are hypothesized to have rapid-onset effects based on secondary analyses and comparisons with ketamine. However, the inconsistency in primary endpoint outcomes suggests that more research is needed to verify these effects and determine the ideal therapeutic window and patient population.
- Safety Profile: While ALTO-202 appears well-tolerated in early trials, the long-term safety profile of GluN2B-NMDA receptor antagonists has yet to be established. As with any new class of medication, the broader implications, risks, and interactions of chronic use are areas of potential uncertainty.
- Preclinical Studies: Animal studies have been pivotal in exploring the potential role of GluN2B-NMDA receptors in depression. For example, transgenic mice with forebrain-specific overexpression of the GluN2B subunit demonstrate depressive-like behaviors, suggesting that dysregulation of GluN2B-containing NMDA receptors may be involved in the development of depression (Tang et al., 2015).
- Neuroimaging Research: Clinical neuroimaging studies using techniques like magnetic resonance spectroscopy (MRS) have reported altered glutamate levels in various brain regions in patients with MDD. These studies have provided indirect evidence linking abnormal glutamatergic neurotransmission involving NMDA receptors with the pathophysiology of depression.
- Clinical Trials of NMDA Receptor Antagonists: The investigation of nonselective NMDA receptor antagonists like ketamine in clinical trials has provided strong evidence for the antidepressant potential of targeting NMDA receptors. These studies have observed rapid and robust antidepressant effects that are often apparent within hours of administration, unlike traditional antidepressants that can take weeks to become effective (Zarate et al., 2006).
- Studies on GluN2B Selective Antagonists: A number of studies have aimed to determine whether selective GluN2B antagonists can replicate the beneficial effects of ketamine while minimizing potential side effects. Research into compounds like traxoprodil (also known as CP-101,606) and MK-0657 has shown promise for the treatment of depression through the selective inhibition of the GluN2B subunit, with some studies showing improvements in depressive symptoms (Preskorn et al., 2008).
- Meta-Analyses and Reviews: Meta-analyses and systematic reviews that assess the overall efficacy of NMDA receptor antagonists in depression often include discussion of the GluN2B subunit's potential role in the therapeutic effects (Krystal et al., 2019).
- Rapid Antidepressant Effects: There is robust clinical evidence demonstrating the fast-acting antidepressant effects of NMDA receptor antagonists, such as ketamine, which provides a strong argument for the involvement of NMDA receptors in MDD.
- Neurobiological Plausibility: The glutamatergic system's role in synaptic plasticity and neuronal connectivity supports the biological plausibility of targeting NMDA receptors for therapeutic effects in MDD.
- Preclinical Models: Animal studies support the involvement of the GluN2B subunit in behaviors related to depression and have shown that GluN2B antagonists can reverse these behaviors.
- Diverse Clinical Data: Early clinical trials with compounds targeting GluN2B-NMDA receptors suggest potential antidepressant effects, supporting the conceptual underpinnings of selectively targeting these receptors.
- Inconsistent Clinical Evidence: Clinical studies have shown mixed results, with some GluN2B-NMDA receptor antagonists not meeting primary endpoints in major trials or showing efficacy that may not be as strong or as rapid as initially hoped based on preclinical or early-phase human studies.
- Side Effects and Safety: While selective GluN2B antagonism is proposed to minimize side effects, the long-term safety and the potential for off-target effects remain concerns, with more data needed to confirm a favorable risk-benefit ratio, especially in comparison to established treatments.
- Limited Large-Scale Trials: Many of the supportive clinical trials are early-phase, small, or not adequately powered to provide definitive evidence. Large-scale, randomized controlled trials are needed to establish efficacy and safety firmly.
- Mechanistic Uncertainty: The exact mechanisms through which NMDA receptor antagonists exert their effects are not fully understood. While it is known that they affect synaptic plasticity and neurotrophic signaling, the critical pathway for the antidepressant effect is still unclear.
- Translational Challenges: Results from animal models and early-phase trials do not always translate into clinical success, and the relevance of findings from preclinical studies to human MDD is uncertain.
- Comparative Effectiveness: Given the existing treatments for MDD, including SSRIs and other traditional antidepressants, the comparative effectiveness, positioning within treatment protocols, and long-term outcomes of GluN2B-NMDA receptor antagonists are yet to be established.
- Incidence and Prevalence of MDD: We need data on how prevalent MDD is in the intended market. For the sake of this exercise, let's assume there are 17 million individuals with MDD.
- Treatment-Seeking Rate: Not every individual with MDD seeks treatment. Let's assume 50% of the individuals seek treatment.
- Diagnosed and Addressable Population: Even among those who seek treatment, not everyone is diagnosed or has access to ALTO-202. Let's assume an 80% diagnosis rate and that 60% of the diagnosed are addressable (due to indication, severity of disease, etc.).
- Market Share and Access: As a new treatment option, penetration into the market takes time. Let's assume a year 1 market share of 2% that grows to 10% by year 5.
- Price of Treatment: The annual cost per patient will depend on the pricing strategy. Here we assume a price of $9,000 annually.
- Gross-to-Net Adjustments: Not all revenue booked is realized due to discounts, rebates, and other adjustments. Assume a 30% rebate/discount rate.
- Insurance Coverage: Assume 90% of the population has insurance coverage, which affects reimbursement rates.
- Duration of Treatment: ALTO-202's treatment duration could be chronic (lifelong), but for simplification, let's assume 1 year of continuous treatment.
- Platform Utilization and Proprietary Approach: Alto Neuroscience uses patient-derived data and rigorous analytics, including machine learning, to identify predictive biomarker signatures. This approach is designed to de-risk drug development by integrating a clearer biological understanding of patient subsets early in the process, which is in stark contrast to the traditional drug development method in central nervous system (CNS) disorders that tends to be hit-or-miss.
- Drug Development Pipeline: The company has several novel small-molecule product candidates, such as ALTO-100 and ALTO-300, that are targeted to specific biomarkers associated with MDD. Their strategy is to conduct trials that are both inclusive and tailored to specific patient populations based on these biomarkers, which has the potential to increase the likelihood of successful clinical outcomes.
- Biomarker-Enriched Populations: Alto Neuroscience focuses on developing drugs for patient populations that are traditionally hard to treat due to high unmet needs. By identifying pilot biomarkers and their pharmacodynamic effects, the company aims to initiate Phase 2 proof-of-concept trials to validate the efficacy of their candidates like ALTO-101 and ALTO-203.
- In-Licensing and Acquisition: Recognizing the high failure rates in CNS drug development, Alto Neuroscience is strategically positioned to leverage its proprietary drug development approach to evaluate and potentially enhance the value of in-licensed or co-developed drugs.
- Partnership Strategy: The company is open to partnering their product candidates with larger pharmaceutical firms to maximize their value, utilizing external expertise for development and commercialization when appropriate.
- New Platform: The platform and approach by Alto Neuroscience are unproven in clinical practice and regulatory environments. Success is contingent on demonstrating that the predictive biomarker signatures can consistently lead to better patient outcomes and that regulatory bodies will accept this evidence for approval purposes.
- Biomarker Discovery: Identifying and validating biomarkers that can predict treatment response with a high degree of accuracy is difficult and complex. Reproducibility and consistency across different patient populations pose further challenges.
- Clinical Trial Design: Trials involving biomarker-enriched populations may face enrollment challenges and may not reflect the broader patient population, possibly limiting generalizability.
- Regulatory Hurdles: The need for companion diagnostics can add layers of complexity to drug approval and market access, as the FDA may require additional validation for these diagnostic tools.
- Commercial Scalability: The strategy's commercial viability relies on the ability to accurately identify and segment patients in routine clinical practice, which may require substantial investment in new technologies and education for healthcare providers.
Keep in mind that the actual revenue will be influenced by many factors including successful completion of clinical trials, regulatory approval, competition, marketing effectiveness, and continued clinical and real-world evidence of efficacy and safety. The figures used in this example are purely hypothetical and intended to illustrate the process of constructing a revenue build; true revenue forecasting would require more specific data and complex models.
To estimate the probability of clinical success at various phases for ALTO-101 in Cognitive Impairment Associated with Schizophrenia (CIAS), we'll use the industry-standard success rates and factor in the positive preclinical data for ALTO-101.
Given industry averages for Neurology products:
The promising results from ALTO-101's Phase 1 trials suggest that it might perform at least as well as the industry averages. However, to be conservative, we will not adjust these probabilities upward, as clinical trial success can often be unpredictable, and positive Phase 1 results do not guarantee success in later phases.
ALTO-203
ALTO-203 is a novel small molecule histamine H3 receptor inverse agonist being developed as a treatment for Major Depressive Disorder (MDD) with a particular focus on anhedonia, which is a symptom characterized by a loss of pleasure or lack of interest in normally rewarding activities.
Therapeutic Rationale:
Taken together, the rationale for pursuing ALTO-203 as a treatment for MDD with anhedonia is to address the underlying neurobiological dysfunction by increasing dopamine release in the brain's reward pathway, thus potentially improving anhedonic symptoms and overall mood. The distinct mechanism of action differentiates it from other treatments and provides a novel approach to managing this challenging aspect of depressive disorders.
The science behind the therapeutic rationale for ALTO-203 in treating Major Depressive Disorder (MDD) with anhedonia involves several well-established as well as emerging concepts in neuroscience and psychopharmacology. Here's a breakdown of the established elements and those areas that may still be under debate or require further research:
Established Science:
Areas of Uncertainty or Debate:
Level of Evidence: The overall level of evidence supporting the processes described involves a combination of preclinical studies, early-stage clinical trials, and pharmacological knowledge. The preclinical evidence showing that ALTO-203 increases dopamine release in the nucleus accumbens is a promising sign for its therapeutic potential. However, higher-quality evidence from randomized controlled trials in humans is essential to confirm its efficacy and safety in treating MDD with anhedonia.
In summary, while the neuropharmacological rationale for ALTO-203 as a treatment for anhedonia in MDD is grounded in an understanding of H3 receptors and dopaminergic signaling, the clinical application of this knowledge is still at a relatively early stage. The science is promising but must be validated through rigorous clinical testing to resolve the uncertainties and scientific debate surrounding the efficacy of H3 inverse agonists in this context.
As of my knowledge cutoff in early 2023, while the histamine H3 receptor (H3R) is an established target for certain central nervous system (CNS) disorders, its role in Major Depressive Disorder (MDD) with anhedonia is more emergent and subject to ongoing research. The H3 receptor functions predominantly as a presynaptic auto- and heteroreceptor in the brain, modulating the release of various neurotransmitters, including histamine, acetylcholine, norepinephrine, and notably, dopamine. Here are a few insights supported by the scientific literature on the potential role of the H3 receptor in MDD and anhedonia:
It's important to note that much of the available data stem from preclinical studies, which necessitates cautious interpretation when translating these findings to human conditions. Additionally, the H3 receptor inverse agonists' effects on neurotransmitter release in brain regions associated with reward processing do not yet have a robust body of clinical data to conclusively establish their efficacy for MDD with anhedonia.
When it comes to systematic reviews, meta-analyses, or large-scale clinical studies focused on H3 receptor inverse agonists for anhedonia in MDD, the literature is not extensive as of yet. The developmental stage of novel drugs targeting this mechanism, such as ALTO-203, suggests that more definitive evidence will emerge from ongoing and future Phase 2 and Phase 3 clinical trials.
In summary, scientific interest in the role of H3 receptors in MDD with anhedonia is grounded in preclinical evidence and pharmacological theory, but there is a significant gap in clinical evidence that needs to be filled to confirm the therapeutic potential of H3 receptor inverse agonists in this specific patient population. As such, research results anticipated in the coming years will be crucial for establishing the clinical relevance of this approach.
The evidence base supporting the therapeutic rationale for using H3 receptor inverse agonists in treating Major Depressive Disorder (MDD) with anhedonia can be evaluated in terms of its strengths and weaknesses:
Strengths of the Evidence Base:
Weaknesses of the Evidence Base:
The level of evidence supporting the therapeutic rationale for H3 receptor inverse agonists in MDD with anhedonia is currently at a stage that suggests promise but requires more empirical validation. The enthusiasm drawn from preclinical data and pharmacological theory needs to be balanced with rigorous clinical trial data to establish efficacy, safety, and the scope of the potential therapeutic benefits for patients with MDD and anhedonia. As such, future research outcomes will be critical in determining the viability of this therapeutic strategy.
Clinical data
The clinical data supporting the use of ALTO-203 for Major Depressive Disorder with anhedonia are derived from Phase 1 trials originally conducted by Cephalon and Teva prior to the licensing of ALTO-203 by the current developer. The following points summarize the clinical data available:
Overall, the clinical data indicate that ALTO-203 is well tolerated and may have beneficial effects on mood and cognitive components relevant to Major Depressive Disorder with anhedonia. However, these findings are preliminary, based on Phase 1 trials in healthy volunteers, and further research is needed to determine the efficacy and safety of ALTO-203 in patients with Major Depressive Disorder.
The approvable endpoints for a novel treatment like ALTO-203 in Major Depressive Disorder (MDD) with anhedonia would typically need to align with regulatory agency requirements such as those from the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Anhedonia, which is the loss of interest or pleasure in normally rewarding activities, is a significant symptom of MDD and represents a particular area of unmet medical need. As such, endpoints would need to measure both the severity of the depression and the degree of anhedonia.
Possible approvable endpoints for ALTO-203 could include:
Clinical studies that would typically be required:
The estimated number of patients required:
For Phase 2 trials, it's common to see anywhere from 100 to 300 patients, depending on the design and objectives. For Phase 3, the number of patients is typically in the range of 300 to 3,000, but this can vary based on statistical power calculations tailored to the expected effect size, variability in response, and the dropout rate, among other factors. It's also common for regulators to require two positive Phase 3 studies for approval, each having adequate sample sizes to ensure the statistical robustness of the data.
In summary, the clinical development of ALTO-203 for MDD with anhedonia will need to incorporate specialized scales that measure both depressive symptoms and specific aspects of anhedonia, involve both intermediate-sized Phase 2 and large Phase 3 trials, and ensure sustained follow-up of patients to track long-term safety and efficacy. The exact number of required patients would be determined by statistical power calculations that would take into account the size of the expected treatment effect and the variability in the patient population.
Market overview
Major Depressive Disorder with Anhedonia
Existing Treatments and Standard of Care:
Currently, the standard of care for MDD includes selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), atypical antidepressants, tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs). Additionally, psychotherapy, lifestyle changes, and other supportive treatments are used.
Medications like SSRIs (e.g., fluoxetine, sertraline) and SNRIs (e.g., venlafaxine, duloxetine) are often first-line treatments. However, the response rates for these drugs can vary, and a significant number of patients do not achieve full remission. Furthermore, existing treatments might not adequately address anhedonia, which is linked to decreased life satisfaction and persistent functional impairments.
Unmet Medical Need:
There is a critical unmet medical need for therapies that directly target anhedonia within the broader context of MDD. Patients with anhedonia often have a poorer response to conventional antidepressants, and this symptom can be a predictor of overall less favorable outcomes. Thus, any therapeutic development that effectively addresses anhedonia would fill a significant treatment gap and potentially improve the prognosis for patients with MDD.
Revenue build
Given the hypothetical scenario for ALTO-203 and the provided clinical background, I'll create a high-level, generalized revenue build for the drug in the Major Depressive Disorder (MDD) with anhedonia space. Please note that these figures will be placeholder estimates and should be refined with more detailed market research, pricing analysis, and input from clinical development.
Target Population Estimation:These projections will need to be regularly updated due to the dynamic nature of the pharmaceutical market, regulatory changes, competitive landscape shifts, and emerging clinical data.
Example Placeholder Estimates:(These are illustrative examples and would require a solid data foundation to be considered actionable)
It's crucial to closely monitor the data from upcoming clinical trials, competitor activity, and market trends to provide continual refinements to these placeholder estimates.
ALTO-202
The therapeutic rationale for using a GluN2B-NMDA receptor antagonist like ALTO-202 in Major Depressive Disorder (MDD) stems from several key points related to the pathophysiology of the disease and the pharmacological action of such antagonists:
In summary, the concept of using a GluN2B-NMDA receptor antagonist like ALTO-202 is founded on the neuropathological evidence of glutamatergic dysfunction in MDD, the successful application of other NMDA antagonists as antidepressants, and the potential for rapid-onset action while maintaining a tolerable safety profile. Further well-powered studies are required to fully determine the efficacy, dosage, and safety parameters for the clinical use of ALTO-202 in treating MDD.
The science behind the role of the glutamatergic system in Depression and the use of NMDA receptor antagonists like ketamine for treatment are fairly established, yet several aspects remain under continued research and debate:
In terms of overall evidence, the foundation of the Dysregulated glutamatergic system's role in MDD and the general mechanism of action of NMDA receptor antagonists are supported by substantial preclinical and clinical data. However, the evidence supporting the advantages of specifically targeting the GluN2B subunit, and the specific effects of ALTO-202, is less robust and requires further validation. Upcoming trials will be critical for establishing the clinical utility of ALTO-202, defining its efficacy, and confirming its safety profile. These will contribute to the growing body of evidence on the role of glutamatergic modulation in MDD treatment.
The literature supporting the role of GluN2B-NMDA receptors in Major Depressive Disorder (MDD) emanates from various sources, including preclinical studies, neuroimaging research, and clinical trials of GluN2B-NMDA receptor antagonists.
While these findings are compelling, it's critical to note that research into the selective blockade of the GluN2B subunit is not as well established as the research into broader NMDA receptor antagonism. Moreover, many of the published studies thus far have been early-phase clinical trials, small in scale, or preclinical research. Larger, well-designed clinical trials are necessary to definitively confirm the specific role of GluN2B-NMDA receptors in MDD and establish a solid evidence base for GluN2B selective antagonists as effective therapeutic agents for the disorder.
The therapeutic rationale for the use of GluN2B-NMDA receptor antagonists in Major Depressive Disorder (MDD) is supported by the convergence of evidence from multiple research avenues. Nonetheless, the evidence base has strengths and weaknesses.
Strengths:
Weaknesses:
In conclusion, the evidence base provides a compelling rationale for the continued investigation of GluN2B-NMDA receptor antagonists in treating MDD. However, it also highlights the need for more rigorous clinical research to overcome the current weaknesses in the evidence and establish these antagonists as a standard part of depression therapeutics.
Financial model
When performing a hypothetical revenue build for a pharmaceutical product like ALTO-202 intended for the treatment of Major Depressive Disorder (MDD), there are key factors to consider. To construct this revenue build, we need to make some assumptions and consider several market variables, including but not limited to the prevalence of the condition, addressable patient population, treatment penetration rates, pricing, duration of treatment, and payer mix.
Assumptions:
Platform strategy
Alto Neuroscience's scientific strategy centers on the development of personalized treatments in neuropsychiatric care by leveraging advances in our understanding of neurobiology and brain-based biomarkers. Their approach seeks to align medication to patient groups based on predictive biomarker signatures, which could improve the effectiveness of treatments for conditions such as major depressive disorder (MDD) and beyond.
Here's an overview of their strategy:
Comparing Alto Neuroscience’s strategy with the precision medicine advances in oncology reveals similarities in the use of targeted therapies based on patient-specific biological markers. However, the application of such a strategy is more nascent in neuropsychiatry.
Risks and Pitfalls:
Overall, while Alto Neuroscience's precision psychiatry approach has the potential to revolutionize the treatment of neuropsychiatric disorders, it also faces significant scientific, clinical, regulatory, and commercial challenges that will need to be addressed as development proceeds.