Boundless Bio IPO investment analysis
March 7, 2024
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or an ongoing business relationship with the company.
Overview
Boundless Bio is a clinical-stage oncology company specializing in innovative cancer therapeutics targeting extrachromosomal DNA (ecDNA) to treat patients with oncogene amplified tumors. Their approach focuses on the root cause of oncogene amplification found in over 14% of cancers, aiming to improve treatment outcomes for these historically challenging cases.
Central to Boundless Bio's strategy is the proprietary Spyglass platform, designed to identify drug targets essential to ecDNA functionality in oncogene amplified cancer cells. The company has developed a portfolio of ecDNA-directed therapeutic candidates (ecDTx), aimed at inhibiting proteins critical for ecDNA maintenance and function. hese small molecule drugs are designed to inhibit key targets crucial for ecDNA functionality, presenting a new paradigm in cancer treatment by preventing cancer cells from using ecDNA to express amplified oncogenes and become resistant to existing therapies.
The leading therapeutic candidate, BBI-355, is a selective inhibitor of checkpoint kinase 1 (CHK1), vital for ecDNA replication and transcription. BBI-355 has demonstrated promising CHK1 inhibition and tumor regression in preclinical models and is currently under Phase 1/2 clinical trials. Another key candidate, BBI-825, targets ribonucleotide reductase (RNR), essential for ecDNA assembly and repair, and has entered Phase 1/2 trials in patients with resistance gene amplifications. The company is also advancing a third ecDTx program focusing on a kinesin essential for ecDNA segregation during cell division, with an Investigational New Drug (IND) application expected by the first half of 2026. These targets represent novel approaches to disrupt cancer's reliance on ecDNA, potentially offering a strategy to overcome the rapid adaptability characterizing oncogene-amplified tumors.
To identify patients likely to benefit from ecDTx, Boundless Bio has developed an ecDNA diagnostic tool, ECHO, using next-generation sequencing to detect ecDNA in tumor samples. This tool has been recognized by the FDA as a non-significant risk device in the POTENTIATE trial, underlining its potential for patient selection.
Since its inception, Boundless Bio has secured significant funding from leading life science investors and leverages unique insights into ecDNA biology and synthetic lethality to expand its pipeline of novel cancer therapies. The company's leadership includes globally recognized experts such as Dr. Paul Mischel, positioning Boundless Bio at the forefront of addressing unmet needs in oncogene amplified cancers.
Boundless Bio's strategic focus on exploiting ecDNA vulnerabilities represents a novel paradigm in cancer treatment. By combining the Spyglass platform, a robust pipeline of ecDTx candidates, and the ECHO diagnostic tool, the company aims to transform therapeutic landscapes for patients facing previously intractable oncogene amplified cancers.
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| BBI-355 | Small molecule | CHK1 Inhibitor | Oncogene amplified cancers | |||||||
| BBI-825 | Small molecule | RNR Inhibitor | MAPK pathway activated cancers |
Highlights and risks
Strong biologic rationale supporting mechanisms of action
Clinical evidence of effectiveness from drugs with similar MOA provides validation to scientific thesis
Targeting specific susceptible patient populations can potentially improve therapeutic window
Stable disease observed in five of eighteen evaluable Phase 1 subjects with one breast cancer patient experiencing significant lesion reduction
Patient selection strategy is crucial to finding workable therapeutic window
Targeting highly competitive markets with challenging clinical development paths
Oncology clinical development is highly risky with low probability of approval
Interim Phase 1 disease control and response rates do not appear to be superior to other CHK1 inhibitors
Valuation
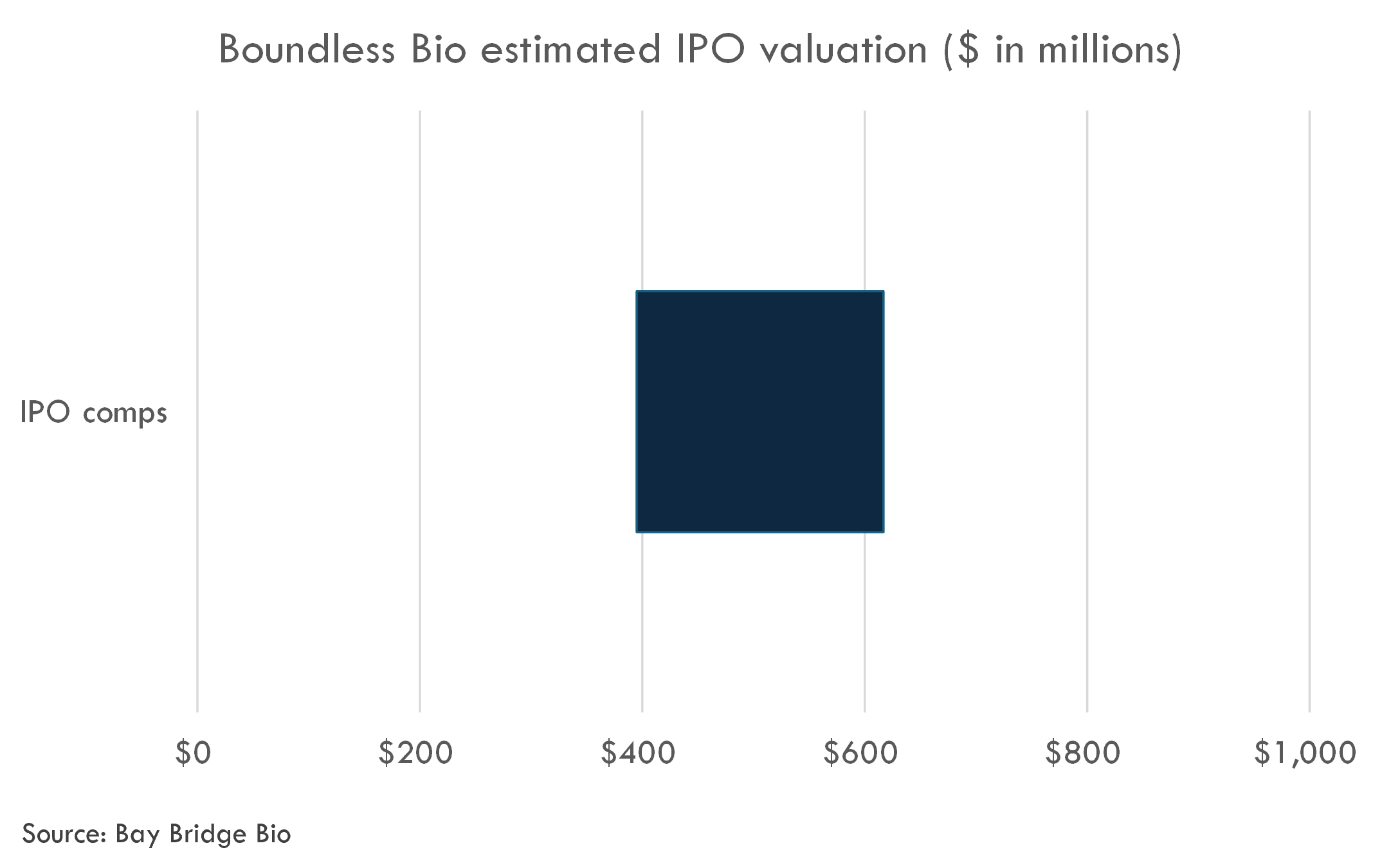
The company filed to go public in March 2024. The post-money valuation of the round, a $100 million Series C in April 2023, is estimated to be $250 million.
We estimate the fully diluted, post-money valuation to be in the range of $395-617 million. Due to the early-stage nature of the company, we did not conduct a DCF analysis. We also did not include M&A comps, because on a probability-adjusted basis, the M&A value is relatively small for Phase 1 oncology companies.
BBI-355
Scientific background
The therapeutic rationale for using a CHK1 (Checkpoint Kinase 1) inhibitor in oncogene-amplified cancers is deeply rooted in the understanding of cellular DNA damage response (DDR) mechanisms and the distinctive vulnerabilities of cancer cells. CHK1 is a serine/threonine-specific protein kinase that plays a crucial role in the DDR pathway, especially in response to DNA replication stress and damage. It is involved in cell cycle arrest, DNA repair, and, if damage is irreparable, triggering apoptosis.
Oncogene amplification in cancers often leads to a state of inherent DNA replication stress due to the unscheduled and accelerated cell division. This stress promotes genomic instability, a hallmark of cancer, making cancer cells more reliant on DDR pathways, including the CHK1-mediated pathway, to survive and proliferate despite accumulating DNA damage. In simple terms, oncogene amplification inadvertently forces cancer cells into a precarious balance, heavily depending on CHK1 to manage the DNA damage without succumbing to it.
Inhibiting CHK1 in oncogene-amplified cancers disrupts this delicate balance. When CHK1 function is compromised by an inhibitor, it leads to an overwhelming accumulation of DNA damage during the DNA replication process. The cancer cells, already vulnerable due to oncogene-induced stress and impaired by the inhibited DNA repair capability, are unable to cope with this added burden. The result is a failure in cell cycle progression, potentially leading to mitotic catastrophe and cell death. Additionally, CHK1 inhibitors can sensitize cancer cells to DNA-damaging agents (such as radiation and certain chemotherapies), making them a valuable tool in combination therapies.
Therefore, the therapeutic rationale behind targeting CHK1 in oncogene-amplified cancers lies in exploiting the increased reliance of such cancer cells on the CHK1-mediated DDR pathway. By inhibiting CHK1, one can push the cells beyond their capacity to manage DNA damage, leading to cancer cell death while sparing normal cells that do not exhibit the same level of DNA replication stress and reliance on CHK1 for survival. This targeted approach underscores the importance of understanding cancer cell vulnerabilities and DDR mechanisms in developing effective cancer therapies.
The science underlying the therapeutic rationale for CHK1 inhibitors in oncogene-amplified cancers is robust, yet it is also an area of active research and ongoing development. Various components of the DNA damage response (DDR) pathways and the role of CHK1 in these processes are well established. However, translating this knowledge into effective cancer therapies involves complexities that are still being unraveled. Key points that are subject to ongoing research, uncertainty, or scientific debate include:
- Cancer Specificity and Selectivity: While CHK1 is a critical mediator in DDR pathways, especially in oncogene-amplified cancers, determining the specificity and selectivity of CHK1 inhibitors is complex. The challenge lies in targeting cancer cells preferentially without causing unacceptable toxicity in normal cells, given CHK1's role in normal cellular function and DNA repair.
- Resistance Mechanisms: Like many targeted therapies, there's a concern that cancers may develop resistance to CHK1 inhibitors. The mechanisms behind such resistance, whether through the mutation of CHK1 itself or through compensatory pathways in the DDR, are areas of active investigation.
- Combination Therapy Strategies: CHK1 inhibitors are thought to be particularly effective in combination with DNA damaging agents or other chemotherapies. However, the optimal combinations, dosing schedules, and sequencing of these therapies to maximize efficacy while minimizing toxicity are still under investigation.
- Biomarkers of Response: Identifying which patients are most likely to benefit from CHK1 inhibitors is crucial. Research is ongoing into biomarkers that can predict response to CHK1 inhibition. This includes not only the presence of oncogene amplification but also indicators of replication stress and other components of the DDR machinery.
- Therapeutic Window and Dosage: Finding the therapeutic window that effectively targets cancer cells while sparing healthy cells is a nuanced challenge. The optimal dosage and administration schedule that achieves this balance require extensive clinical trials to establish.
Overall, the level of evidence supporting the processes described is substantial, particularly in pre-clinical models and early-stage clinical trials. The theoretical foundation, based on the biology of DDR and the role of CHK1, is strong. However, translating these findings into safe and effective treatments for humans is a complex process that necessitates further research. The number of ongoing clinical trials targeting CHK1 in various oncogene-amplified cancers will provide critical data to clarify these uncertainties and potentially validate the therapeutic rationale of CHK1 inhibitors in clinical settings.
Multiple studies and literature reviews have supported the role of CHK1 in oncogene-amplified cancers, underscoring its potential as a therapeutic target. This evidence comes from various sources including preclinical studies, cell line experiments, and early clinical trials. Below are some examples that highlight CHK1's significance in this context:
- Preclinical Studies: Several preclinical studies have demonstrated that CHK1 is crucial for the survival of cancer cells experiencing oncogene-induced replication stress. An example can be found in the work by Sørensen et al. (Nature, 2012), which showed that CHK1 inhibition led to increased DNA damage and cell death in cancer cells under replication stress, suggesting a vulnerability that can be exploited therapeutically.
- CHK1 and Oncogene Interactions: Research has indicated that certain oncogenes, like MYC and RAS, which are often amplified in various cancers, induce replication stress that makes cancer cells more dependent on CHK1 for survival. King et al. (Molecular Cell, 2015), for instance, provided evidence that MYC-overexpressing cells are particularly sensitive to CHK1 inhibitors due to this induced replication stress.
- Clinical Trials with CHK1 Inhibitors: Although still in the early stages, clinical trials involving CHK1 inhibitors have begun to provide insight into their efficacy in cancer therapy, particularly in combination with chemotherapeutics or other DDR inhibitors. Trials are exploring the response of various cancers with oncogene amplification to CHK1 inhibition, aiming to confirm the preclinical observations in human subjects. Information on these trials can typically be found on clinical trial databases and through publications related to specific trials.
- Combination Therapy Insights: Studies suggest that CHK1 inhibitors can synergize with other treatments, such as DNA-damaging agents, to preferentially kill cancer cells. This is partly because CHK1 inhibition can exacerbate the effects of DNA damage in cells with already compromised repair mechanisms due to oncogene amplification. Preclinical work, such as that by Morgan et al. (Cancer Research, 2010), supports the combination of CHK1 inhibitors with chemotherapeutics for enhanced efficacy.
- Mechanistic Studies on DDR Pathways: Fundamental research into DDR pathways has clarified CHK1's role, not just in cell cycle regulation, but also in controlling the halting of replication forks and facilitating repair, which is crucial in the context of oncogene-induced stress. Insights into these mechanisms are detailed in reviews and studies focusing on the intricacies of the DDR machinery.
It is important to note that while the rationale and preliminary data are promising, the transition from preclinical to clinical success involves overcoming significant hurdles, including drug resistance, toxicity, and patient selection. Hence, the ongoing research and results from forthcoming clinical trials are crucial to fully understanding CHK1's role in oncogene-amplified cancers and its potential as a therapeutic target.
The therapeutic rationale for targeting CHK1 in oncogene-amplified cancers is supported by a substantial evidence base that spans preclinical studies, cell line experiments, and early clinical trials. Here's an assessment of the strengths and weaknesses of this evidence base:
Strengths
- Biological Plausibility: The biology underpinning CHK1's role in the DNA damage response (DDR) and cell cycle regulation is well-established. This provides a strong theoretical basis for why inhibiting CHK1 might be particularly effective in cancers burdened with oncogene-induced replication stress.
- Preclinical Evidence: A broad range of preclinical studies have demonstrated that CHK1 inhibition can lead to enhanced DNA damage, cell cycle arrest, and apoptosis in cancer cells, particularly those exhibiting oncogene amplification. These studies provide a strong foundation for the hypothesis that CHK1 inhibitors can be effective anticancer agents.
- Synergy with Other Therapies: There is well-documented evidence that CHK1 inhibitors can act synergistically with other treatments, such as DNA-damaging agents. This synergy could allow for lower doses of chemotherapeutics, potentially reducing side effects while maintaining or enhancing anticancer efficacy.
- Technological Advancements: Advances in genomic technologies have made it easier to identify patients with oncogene-amplified cancers who are most likely to benefit from CHK1 inhibitors, supporting a precision medicine approach to treatment.
Weaknesses
- Translation from Preclinical to Clinical: While preclinical studies are promising, the translation of these findings to clinical success has been challenging. Differences in tumor biology between model systems and human cancers can lead to discrepancies in drug efficacy and toxicity profiles.
- Complexity of Cancer Biology: Cancer's genetic heterogeneity and the ability of cancer cells to adapt and develop resistance mechanisms pose significant challenges to the long-term effectiveness of CHK1 inhibitors. The complexity of these adaptive responses isn't fully understood and remains a significant barrier to developing universally effective treatments.
- Side Effects and Toxicity: As CHK1 plays a role in normal cell functions, particularly in the DDR, there is a potential for side effects stemming from the systemic inhibition of CHK1. The full spectrum of these side effects in humans is not yet completely understood and represents a weakness in the current evidence base.
- Optimal Combination Strategies: While combining CHK1 inhibitors with other therapies is a promising strategy, determining the most effective combinations, dosages, and treatment schedules requires complex, time-consuming clinical trials. There's still much to learn about how best to integrate CHK1 inhibitors into broader cancer treatment regimens.
- Biomarker Development: The identification and validation of predictive biomarkers for response to CHK1 inhibitors are ongoing. Without robust biomarkers, tailoring CHK1 inhibitor therapy to individual patients remains a challenge, potentially limiting the effectiveness of these treatments in a broader patient population.
In summary, while there is a strong theoretical and preclinical rationale for targeting CHK1 in oncogene-amplified cancers, translating these insights into effective, safe, and universally applicable clinical treatments remains a work in progress. Ongoing research into cancer biology, drug resistance mechanisms, and patient stratification will be key to overcoming these challenges.
Clinical trial overview
Study Design Summary of BBI-355 Development
The clinical study revolves around BBI-355, a novel oral, potent, and selective checkpoint kinase 1 (CHK1) inhibitor targeting extrachromosomal DNA (ecDNA) in oncogene-amplified cancers. This first-in-human study is structured in three parts, aiming to define the safety profile, maximum tolerated dose (MTD), and recommended Phase 2 dose (RP2D) of BBI-355 both as a standalone therapy and in combination with selected targeted therapies. The study targets a suite of advanced solid tumors resistant or unsuitable for existing standard therapies.
The Phase 1/2 study, termed an open-label, multicenter trial, enrolls subjects with an array of solid tumors such as various forms of cancers (lung, head and neck, esophageal, gastric, breast, bladder, ovarian, endometrial, and liposarcoma). The interventions include BBI-355 as a single agent and in combination with either the EGFR inhibitor erlotinib or the FGFR1-4 inhibitor futibatinib, administered in 28-day cycles.
Primary outcomes include evaluating the treatment-emergent adverse events (TEAEs), determining the MTD and/or RP2D. Secondary outcomes focus on pharmacokinetic measures like C_max, C_trough, T_max, AUC, and anti-tumor efficacy assessed via RECISTv1.1 criteria.
Critiques of the Study Design
- **Open-Label Nature**: The lack of blinding could introduce bias in reporting and assessment of outcomes, particularly subjective ones like side effects.
- **Non-Randomized Allocation**: While common in early-phase trials, this could limit the ability to attribute observed effects directly to the studied therapy, affecting the robustness of conclusions drawn regarding efficacy and safety.
- **Sample Size**: With an enrollment target of 150 participants, while sufficient for early-phase trials, future studies will need larger, more diverse populations to validate findings and ensure generalizability.
- **Sequential Assignment**: This approach is logical for dose-finding studies but may face operational challenges, such as ensuring timely recruitment and managing cohorts receiving different doses or combinations.
Operational or Technical Challenges
- **Participant Recruitment**: Targeting subjects with specific oncogene amplifications requires precise diagnostic capabilities and screening, which can be time-consuming and costly.
- **Dose Escalation and Expansion**: Managing the logistical aspects of adjusting doses and monitoring for adverse reactions, in real-time, demands significant coordination among clinical teams.
- **Combination Therapy Evaluation**: Testing BBI-355 with other inhibitors adds complexity, necessitating careful monitoring of drug-drug interactions and cumulative toxicity.
- **Pharmacokinetic/Pharmacodynamic (PK/PD) Analysis**: The detailed assessment of drug concentrations and their effects, especially in combination therapy arms, requires sophisticated analytical techniques and could encounter variabilities in drug metabolism among participants.
- **Safety Monitoring**: As this is a first-in-human study, there will be heightened vigilance around identifying adverse effects, necessitating robust mechanisms for rapid reporting, evaluation, and response.
Final Thoughts
The carefully structured study design of BBI-355, addressing an unmet need in oncogene-amplified cancers, represents an important step in oncology. Despite the inherent challenges and critiques of early-phase trials, this study has the potential to offer critical insights into the therapeutic value and safety profile of BBI-355. Meeting these operational and technical challenges head-on will be crucial for the successful execution of the study and for advancing BBI-355 through the pipeline of cancer therapeutics.
Potential of the Study for Proof-of-Concept
The study is designed to evaluate the efficacy and safety of BBI-355, a checkpoint kinase 1 (CHK1) inhibitor, in patients with oncogene-amplified cancers. The proof-of-concept aims to demonstrate that blocking CHK1 can prevent the proliferation of cancer cells with oncogene amplification, a premise supported by the choice of primary and secondary endpoints and the tightly defined inclusion and exclusion criteria.
Appropriateness of Primary and Secondary Endpoints
**Primary Endpoints:**
- **Frequency and severity of treatment-emergent adverse events (TEAEs):** This endpoint is crucial for a first-in-human trial, as it prioritizes patient safety and helps determine the maximum tolerated dose (MTD) and/or recommended Phase 2 dose (RP2D). The use of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) ensures standardized reporting of adverse effects.
- **MTD and/or RP2D of BBI-355:** Identifying these doses is essential for advancing the drug into later phases of clinical trials while ensuring safety and efficacy against oncogene-amplified cancers.
**Secondary Endpoints:**
- **Pharmacokinetics (PK) measures (Cmax, Ctrough, Tmax, AUC):** These are appropriate for understanding the drug's behavior in the body, which is critical for dose optimization and predicting therapeutic outcomes.
- **Anti-tumor activity (according to RECISTv1.1):** This directly addresses the drug’s efficacy in reducing tumor size, providing direct evidence of its potential as a treatment for oncogene-amplified cancers.
Inclusion/Exclusion Criteria
The eligibility criteria are carefully selected to isolate the effect of BBI-355 in a specific patient population who can potentially benefit from this therapy. Requiring evidence of oncogene amplification directly aligns with the drug’s targeted mechanism of action. Also, mandatory availability of Formalin-Fixed Paraffin-Embedded (FFPE) tumor tissue supports genetic analyses of oncogene amplifications.
**Reproducibility Challenges:**
- **Specific Oncogene Amplification Requirements:** While necessary for a targeted approach, these requirements may limit patient recruitment, especially in cases where the amplification status of EGFR or FGFR is not standardly assessed or due to variable accessibility to comprehensive genomic profiling in different regions or institutions.
- **Prior Exposure to CHK1, EGFR, or FGFR Inhibitors:** Excluding patients with previous exposure to these inhibitors is logical but may complicate replicating the study in real-world settings where such treatments might be more commonly administered, possibly reducing the generalizability of study results.
- **Performance Status Requirement (ECOG PS 0 or 1):** While this ensures participants are fit for the study, it may not reflect the broader population of patients with advanced cancer, many of whom may present with more diminished performance status due to disease progression.
Overall, the Proof-of-Concept Potential
The study design, with its well-defined endpoints and inclusion/exclusion criteria, provides a strong framework for evaluating the proof-of-concept for BBI-355 in oncogene-amplified cancers. The precise targeting based on genetic amplifications should help elucidate the therapeutic potential of BBI-355. However, the stringent eligibility criteria, although necessary for a focused study, might pose challenges in patient recruitment and could impact the broader applicability and reproducibility of the findings. Adapting the study design to overcome or adjust for these challenges in future trials will be crucial for confirming the drug's efficacy and safety in a wider patient population.
Interim data update
The company has presented preliminary results for BBI-355, an investigational drug being tested in a Phase 1/2 clinical trial for patients with oncogene-amplified cancers. Here's a detailed summary based on the provided information:
Clinical Development Plan Overview:
- Trial Name and Design: The POTENTIATE trial (NCT05827614) is an open-label, multicenter, first-in-human Phase 1/2 study evaluating BBI-355 alone and in combination with selected targeted therapies in subjects with locally advanced or metastatic solid tumors with oncogene amplifications. The trial aims to enroll 50 to 90 patients to provide preliminary safety and antitumor activity data.
- Patient Population: The trial targets patients with solid tumors that have specific oncogene amplifications (EGFR, FGFR1-4, or CDK4/6), which are known to drive cancer progression. These patients have progressed despite standard therapies or have no further standard treatment options.
- Trial Parts and Modules: The trial is divided into three parts:
- Part 1 focuses on dose escalation and expansion of BBI-355 as a single agent.
- Parts 2 and 3 explore BBI-355 in combination with EGFR, FGFR, or CDK4/6 inhibitors across three modules, aiming to identify the maximum tolerated dose (MTD) and recommended Phase 2 dose (RP2D).
Preliminary Results from Part 1:
- Patient Enrollment and Dosing: Preliminary data come from 22 patients across six dosing cohorts, with doses ranging from 20 mg to 120 mg under different regimens (every other day and 2 days on/5 days off).
- Pharmacokinetics (PK) and Pharmacodynamics (PD): BBI-355 showed good oral bioavailability with dose-proportional increases in peak concentration (Cmax) and area under the curve (AUC). The drug's half-life was around 40 hours, with 2 to 3-fold accumulation at steady state. PD analysis indicated CHK1 target engagement.
- Antitumor Activity: Stable disease was observed in five out of eighteen evaluable subjects, including a significant lesion reduction in a metastatic breast cancer patient.
- Safety and Tolerability: The drug was generally well-tolerated at lower doses without dose-limiting toxicities (DLTs). The 80 mg dose exceeded the target toxicity rate, establishing 60 mg as the MTD for the every-other-day regimen. Common adverse events included decreased neutrophil count, fatigue, nausea, and decreased platelet count.
Key Takeaways and Next Steps:
- The initial phase of the POTENTIATE trial shows promising safety, PK, and preliminary efficacy of BBI-355, warranting further investigation.
- The study has advanced to Part 2, with dose escalation plans based on preclinical and early clinical data.
- Collaborations with Taiho Oncology and Eli Lilly are in place for the supply of targeted therapies for combination treatment modules.
- Future steps include engaging with regulatory bodies for potential registrational paths based on observed antitumor activity and safety profiles.
This summary reflects the trial's scope, focusing on identifying effective treatments for oncogene-amplified cancers, a high unmet need area. The results thus far indicate BBI-355's potential as a new therapeutic option, pending further data from ongoing and future study parts.
Weekly analyses of biotech startups, generated by AI
Receive high quality, AI-generated analyses of biotech startups, public companies and scientific papers each week.
Market overview
Oncogene amplified cancers
Oncogene amplified cancers relate to a category of cancers characterized by the overexpression of one or more oncogenes due to amplification at the genetic level. Oncogenes are genes that have the potential to cause cancer, and when they are amplified, their expressions significantly increase, leading to the rapid growth and spread of cancer cells. This abnormality can affect various pathways involved in cell proliferation, differentiation, and survival, which are critical in cancer development and progression.
Pathology
The pathology of oncogene amplified cancers varies depending on the type of oncogene involved and the tissue or organ affected. Commonly amplified oncogenes include HER2 (Human Epidermal growth factor Receptor 2) in breast and stomach cancers, MYCN in neuroblastoma, EGFR (Epidermal Growth Factor Receptor) in non-small cell lung cancer, and many others.
Cancers harboring oncogene amplifications typically demonstrate aggressive behavior, with rapid growth, potential to metastasize, and resistance to standard therapies. The oncogenic amplification disrupts normal cellular processes, leading to uncontrolled cell division, evasion of apoptosis (programmed cell death), angiogenesis (formation of new blood vessels to supply the tumor), and invasion through tissues.
Symptoms
Symptoms of oncogene amplified cancers are not specific to the amplification status but rather to the location and type of the cancer. For instance, breast cancers with HER2 amplification may present with a lump in the breast, changes in breast shape or size, and skin changes. Non-small cell lung cancers with EGFR amplification may cause coughing, wheezing, shortness of breath, and chest pain.
Prognosis
The prognosis of oncogene amplified cancers generally tends to be poorer compared to non-amplified cancers because of their aggressive nature and resistance to conventional therapies. However, the emergence of targeted therapies has significantly improved the outcome for some of these cancers. For example, cancers with HER2 amplification can be targeted with drugs like trastuzumab, while EGFR mutations can be targeted with tyrosine kinase inhibitors like gefitinib.
Treatment
Treatment strategies for oncogene amplified cancers often involve a combination of therapies, including surgery, radiation, chemotherapy, and targeted therapy. The latter has become a cornerstone in treating these cancers, offering a more personalized approach by directly inhibiting the oncogenic protein or its downstream effects.
In summary, oncogene amplified cancers present significant challenges due to their aggressive nature and resistance to traditional treatments. However, advances in targeted therapy and personalized medicine are improving outcomes for patients with these conditions. An understanding of the specific oncogenic alterations facilitates the development of more effective, less toxic treatments and highlights the importance of genetic testing in the management of cancer patients.
To assess the market opportunity for BBI-355 in oncogene amplified cancers, a thorough understanding of the current treatment landscape, existing successful drugs, the standard of care, and the unmet medical needs in this domain is imperative. Oncogene amplified cancers constitute a segment where precision medicine has significantly transformed treatment paradigms, offering tailored therapeutic approaches based on specific genetic alterations.
Existing Successful Drugs
Several successful drugs have been developed targeting oncogene amplified cancers, setting high standards in the market:
- Trastuzumab (Herceptin): A monoclonal antibody targeting HER2-positive breast cancers, pioneering personalized medicine in oncology.
- Osimertinib (Tagrisso and other TKIs: Tyrosine kinase inhibitors (TKIs) targeting EGFR mutations in non-small cell lung cancer.
- Crizotinib (Xalkori): Targets ALK (anaplastic lymphoma kinase) rearrangements in non-small cell lung cancer.
These drugs have revolutionized treatment, offering significant survival benefits but also denote the high market competition and the regulatory precedent for approval in these indications.
Standard of Care
The standard of care for oncogene amplified cancers involves a combination of surgery, chemotherapy, radiation therapy, and increasingly, targeted therapies and immunotherapies based on the tumor’s genetic profile. Molecular diagnostics have become integral in guiding treatment decisions, with targeted therapy representing a significant portion of the treatment regimen for patients with identifiable genetic mutations.
Unmet Medical Need
Despite the advancements, there remain substantial unmet needs:
- Resistance to Current Therapies: Many patients eventually develop resistance to existing targeted therapies, underscoring the need for new treatment options.
- Limited Treatment Options for Certain Amplifications: Not all oncogene amplifications currently have effective targeted therapies, leaving a significant patient population with limited treatment options.
- Toxicity and Side Effects: Currently available treatments can have considerable side effects, affecting patient quality of life and limiting compliance with the therapeutic regimen.
Competing CHK1 inhibitors
Acrivon's ACR-368 (prexasertib)
ACR-368, also known as prexasertib, is being developed for the treatment of patients with advanced solid tumors, specifically ovarian, endometrial, and bladder cancers. It has shown promising clinical activity in over 400 patients treated at the recommended phase 2 dose (RP2D) across various clinical trials, including those conducted by its previous sponsor, Lilly, and in investigator-initiated trials at the National Cancer Institute (NCI) and MD Anderson Cancer Center (MDACC). ACR-368's tolerability profile is noted for reversible, manageable hematological toxicities and limited non-hematological toxicities, leading to less than 2% drug-related discontinuations.
- Clinical Activity and Safety: ACR-368 has demonstrated significant anti-tumor activity, including complete responses (CRs), especially in patients with high-grade serous ovarian cancer and squamous cell carcinoma (SCC), primarily those resistant to platinum-based therapies. It has been well-tolerated with manageable toxicities across trials.
- Enhancing Response Rates: By pairing ACR-368 with the OncoSignature test, there's an anticipation of significantly increasing the overall response rate (ORR) by targeting treatment to patients most dependent on CHK1/2. Preliminary expectations suggest 30% to 40% of patients in key indications could be positive for the ACR-368 OncoSignature, potentially leading to a notable increase in ORR.
- Phase 1a/b Trial in SCC: Led by Dr. David Hong at MDACC, this 146-patient multicenter trial aimed to establish the RP2D and assess the clinical activity of ACR-368 in refractory or recurrent SCC, including squamous cell carcinoma of the head and neck (SCCHN), non-small cell lung cancer (sqNSCLC), and anal cancer. The RP2D was set at 105 mg/m2 with an ORR of 5% in SCCHN and 15% in anal cancer, indicating significant efficacy, especially in HPV-positive patients.
- Phase 2 NCI Trial in Ovarian Cancer: This trial led by Dr. Lee at NCI enrolled 28 women with high-grade serous ovarian cancer, achieving an ORR of 29% in the intention-to-treat population, with durable responses over ten months. This highlighted ACR-368's potential in treating platinum-resistant ovarian cancer.
- Phase 2 Multicenter Trial in Ovarian Cancer: Conducted across 46 centers in eight countries, this trial involved patients with platinum-resistant and platinum-refractory ovarian cancer, achieving a 12.1% ORR in 140 patients with platinum-resistant disease. The disease control rate (DCR) exceeded 30% across all cohorts, indicating the durability of responses and overall survival benefit.
Despite the promising activity, the identification of biomarkers predictive of response to ACR-368 has been challenging, with no strong correlation observed between genetic changes or expression of potential biomarker genes and clinical response. This underscores the importance of developing alternative patient responder identification methods, such as the OncoSignature test.
The ongoing Phase 2 trials aim to validate the efficacy of ACR-368 in combination with the OncoSignature test, with hopes of achieving single-agent, single-arm approval for targeted patient populations.
OncoSignature assay
The OncoSignature Assay, tailored for ACR-368 (Prexasertib), a checkpoint kinase 1/2 inhibitor, has been developed to predict treatment sensitivity in cancer patients. This quantitative, multiplexed immunofluorescent assay evaluates three specific biomarkers to determine a patient's likelihood of responding to ACR-368 treatment. Key findings from the validation studies highlight the assay's potential in enhancing treatment efficacy:
- Clinical Effectiveness of ACR-368: ACR-368 has shown durable single-agent activity in various cancers, including high-grade serous ovarian, anal, and head & neck cancers. Previous clinical trials have demonstrated a proportion of patients experiencing significant tumor response rates.
- OncoSignature Assay Predictive Capability: The assay was found to have excellent concordance between predicted ACR-368 responder prevalence and observed overall response rates (ORR) in clinical trials. It identified endometrial and bladder cancers as new sensitive tumor types, predicting 30-40% of cases as ACR-368 responsive.
- Validation in PDX Models and Clinical Biopsies: The assay successfully predicted treatment sensitivity in endometrial cancer PDX models with an AUC of 0.88 in blinded studies. Furthermore, blinded, prospectively-designed studies on pretreatment tumor biopsies from past Phase 2 trials in ovarian cancer patients showed a statistically significant segregation of responders from non-responders, with ORRs of 47% and 58%, respectively, and increased median progression-free survival (mPFS).
- Application in Clinical Trials: The assay involves image analysis and quantitative measurements of the three biomarkers in tumor cell nuclei, determining positive or negative OncoSignature status based on biomarker score thresholds. This method has been validated in cancer cell lines, PDX models, and pretreatment tumor biopsies across various cancers, proving its utility in predicting ACR-368 sensitivity.
- Clinical Trial Outcomes Based on OncoSignature Status: A clinical trial (NCT05548296) is underway, recruiting patients with platinum-resistant ovarian, endometrial, and urothelial cancer, to evaluate ACR-368 efficacy based on OncoSignature test status. Initial studies have shown that OncoSignature-positive patients exhibit significantly better tumor shrinkage and progression-free survival compared to OncoSignature-negative patients.
In summary, the OncoSignature Assay represents a significant advancement in personalized medicine for cancer treatment, enabling the identification of patients likely to benefit from ACR-368 therapy. This approach promises to refine treatment strategies and improve clinical outcomes for patients with various cancer types.
Publications
Lancet Oncology
The study published in Lancet Oncology evaluates prexasertib (LY2606368), an inhibitor targeting cell cycle checkpoint kinases 1 and 2, focusing on its efficacy in treating BRCA wild-type recurrent high-grade serous ovarian carcinoma, a condition marked by TP53 mutations, DNA repair issues, and genomic instability. Conducted as an open-label, single-centre, phase 2 trial, the research aimed to test prexasertib's effectiveness in a specific patient group: women 18 or older with measurable, recurrent high-grade serous or endometrioid ovarian carcinoma, who either had no family history of hereditary breast and ovarian cancer or were confirmed to have BRCA wild-type status.
The participants, numbering 28 women with a median age of 64, underwent treatment with prexasertib, receiving doses intravenously every 14 days in 28-day cycles. The treatment continued until disease progression, the emergence of unacceptable side effects, or withdrawal of consent. The primary measure of success was the investigator-assessed tumor response, adhering to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
- Out of 24 assessable patients, 8 (33%) exhibited partial responses to the treatment, indicating a significant reduction in tumor size.
- The treatment was associated with several high-grade (3 or 4) adverse events, notably neutropenia in 93% of patients, low white blood cell count in 82%, thrombocytopenia in 25%, and anemia in 11%.
- Grade 4 neutropenia occurred in 79% of patients following the first dose but was transient and managed without the need for growth-factor support.
The study concludes that prexasertib demonstrates clinical activity and tolerability in patients with BRCA wild-type high-grade serous ovarian carcinoma, especially among those with platinum-resistant or refractory disease, suggesting its potential for further development in this area. The research was funded by the Intramural Research Program of the National Institutes of Health and National Cancer Institute.
Clinical Cancer Research
The study published in Clinical Cancer Research focuses on the evaluation of prexasertib, a checkpoint kinase 1 inhibitor, specifically in patients with advanced squamous cell carcinoma (SCC). This research was an extension of a phase I study that showed promise for prexasertib as a single-agent therapy for advanced SCC. The drug was administered at a dose of 105 mg/m^2 via a 1-hour infusion on the first day of each 14-day cycle, with patient cohorts categorized by tumor type and treatment line.
- Patient Demographics: The study involved 101 patients, including 26 with SCC of the anus, 57 with SCC of the head and neck (SCCHN), and 16 with squamous cell non-small cell lung cancer (sqNSCLC). These patients were heavily pretreated, with 49% having received three or more prior treatment regimens.
- Safety and Adverse Events: The most common treatment-related adverse event was grade 4 neutropenia, affecting 71% of the patients. Febrile neutropenia was reported in 12% of the patients, indicating a notable but manageable safety profile for prexasertib.
- Efficacy: Median progression-free survival was varied across different types of SCC: 2.8 months for SCC of the anus, 1.6 months for SCCHN, and 3.0 months for sqNSCLC. The clinical benefit rate at 3 months (including complete response, partial response, and stable disease) was 29% across all tumors, with specific rates of 23% for SCC of the anus, 28% for SCCHN, and 44% for sqNSCLC. Notably, four patients with SCC of the anus experienced partial or complete responses, yielding an overall response rate (ORR) of 15%, and three patients with SCCHN had partial responses (ORR = 5%).
- Biomarker Analysis: The study also explored biomarkers related to genes that alter the DNA damage response or increase replication stress, though specific findings from this analysis were not detailed in the abstract.
- Conclusion: Prexasertib was found to have an acceptable safety profile and demonstrated single-agent activity in patients with advanced SCC. The confirmed maximum-tolerated dose of 105 mg/m^2 was recommended for phase II dosing.
This research highlights the potential of prexasertib in treating advanced SCC, especially in heavily pretreated patients, and supports its further development in phase II trials.
Espera's LY2880070
The study presents a Phase I/II trial investigating the oral checkpoint kinase 1 (Chk1) inhibitor LY2880070 (LY), in combination with low-dose gemcitabine (LD GEM), in patients with advanced or metastatic high-grade serous ovarian cancer (HGSOC). Sponsored by Esperas Pharma Inc., the research aimed to explore the safety, pharmacokinetics, and anti-tumor activity of this combination therapy.
The open-label, multi-center study was divided into two parts, with an expansion cohort of 27 patients with HGSOC. Participants received LY (50 mg twice daily for 5 days a week) alongside LD GEM (100 mg/m2 on days 1, 8, and 15) in a 21-day cycle. The study's primary objectives were to characterize dose-limiting toxicities (DLTs) and the overall safety profile of LY+LD GEM in HGSOC patients and to evaluate the combination's anti-tumor efficacy.
The combination therapy was generally well-tolerated, with the most common adverse events being fatigue, nausea, vomiting, diarrhea, fever, dyspnea, neutropenia, and thrombocytopenia. Over 40% of ovarian cancer patients experienced vomiting, nausea, anemia, fever, decreased appetite, elevated ALT, abdominal pain, and fatigue as treatment-emergent adverse events. Documented DLTs included reduced platelet count (Grade 2), fatigue (Grade 3), diarrhea (Grade 3), and thrombocytopenia (twice, Grade 2).
In terms of effectiveness, two patients (7.4%) experienced a partial response (PR) to the treatment, while fourteen patients (51.9%) had stable disease, including one with an unconfirmed PR. In total, eleven patients (40.7%) achieved disease control for ≥ 12 weeks.
The study concluded that the LY and LD GEM combination was generally tolerable for patients with advanced or metastatic HGSOC. However, further research is necessary to identify biomarkers predictive of response in this patient group, indicating a potential for tailored therapeutic strategies. The study is registered under clinical trial number NCT02632448 and was funded by Esperas Pharma Inc.
Preclinical programs
Several companies, including BenevolentAI, Fosun Pharma, and Impact Therapeutics are developing CHK1 inhibitors that are currently in preclinical development.
Comparing clinical data
Comparing the preliminary results of BBI-355 from the POTENTIATE trial with the reported outcomes for ACR-368 (prexasertib) offers an interesting perspective on the development and potential of CHK1 inhibitors in treating advanced solid tumors. Here's an overview of their comparative analysis:
Efficacy and Clinical Activity
- BBI-355 demonstrated preliminary antitumor activity with stable disease observed in five out of eighteen RECIST evaluable subjects, including a significant lesion reduction in a patient with metastatic breast cancer.
- ACR-368 has shown significant anti-tumor activity with complete responses, particularly in high-grade serous ovarian cancer and squamous cell carcinoma (SCC), including those resistant to platinum-based therapies. Its efficacy is further highlighted by specific response rates in trials, such as a 29% ORR in a Phase 2 trial for ovarian cancer and targeted efficacy in HPV-positive SCC patients.
Safety and Tolerability
- BBI-355 was generally well-tolerated at lower dose levels without severe dose-limiting toxicities (DLTs) up to the 60 mg dose, administered every other day. Adverse events were manageable and consistent with the on-target effects expected from CHK1 inhibitors.
- ACR-368 also exhibited a tolerable safety profile with reversible, manageable hematological toxicities, and limited non-hematological toxicities. The drug-related discontinuation rate was less than 2%, indicating a favorable tolerability profile.
Dosing and Administration
- BBI-355 has been tested in varying doses and schedules, including an every-other-day regimen and a 2 days on/5 days off regimen, to find the optimal dosing strategy.
- ACR-368's RP2D was established at 105 mg/m2 in specific cancer types, showcasing a more traditional dose escalation approach to identify the optimal dosing level.
Pharmacokinetics and Pharmacodynamics
- The details on ACR-368's pharmacokinetics and pharmacodynamics were not explicitly provided, unlike BBI-355, where good oral bioavailability and CHK1 target engagement were demonstrated.
Biomarker-Driven Approaches
- ACR-368 integrates the OncoSignature test, aiming to significantly increase overall response rates by identifying patients most likely to benefit from CHK1/2 inhibition. This precision medicine approach is anticipated to enhance response rates in key indications.
- BBI-355 does not mention the use of a specific biomarker-driven selection tool in the preliminary results, focusing instead on the broad application across tumors with oncogene amplifications.
Future Directions and Challenges
- BBI-355 is advancing into further trial phases with the intention to explore combination therapies and potential expansion based on ongoing research and the dynamic clinical landscape.
- ACR-368 faces the challenge of identifying predictive biomarkers for response, although it is moving forward with trials to validate the efficacy of ACR-368 in combination with the OncoSignature test for targeted patient populations.
Conclusion
Both BBI-355 and ACR-368 exhibit promising potential in treating advanced solid tumors through CHK1 inhibition, each with unique aspects of efficacy, safety, dosing strategies, and approaches to patient selection. ACR-368 shows substantial promise with significant clinical activity and a mature development program. In contrast, BBI-355 is still in early development stages but has demonstrated encouraging preliminary results and a tolerable safety profile. The future development of these inhibitors will likely emphasize the importance of precision medicine in enhancing response rates and targeting therapies to patients most likely to benefit.
Broader competitive landscape
Given the competitive landscape of oncology, particularly in the realm of oncogene amplified cancers, several promising treatments are in various stages of development. These emerging therapies, leveraging novel mechanisms of action and targeting unmet medical needs, could potentially compete with BBI-355. It's crucial to consider treatments that are making significant progress due to their innovative approaches, including small molecule inhibitors, monoclonal antibodies, antibody-drug conjugates (ADCs), and cell therapy strategies. Below, we explore a few examples based on their mechanism of action and targeted oncogenes:
Small Molecule Inhibitors
- Next-Generation Tyrosine Kinase Inhibitors (TKIs): Several companies are developing next-generation TKIs designed to overcome resistance mechanisms that limit the efficacy of first-generation drugs. These include TKIs with less susceptibility to mutation-induced resistance, offering a competitive stance against BBI-355 if it targets similar pathways.
- PI3K/AKT/mTOR Pathway Inhibitors: Given the pivotal role of the PI3K/AKT/mTOR pathway in cancer cell growth and survival, new inhibitors targeting this pathway could provide effective treatment options for patients with oncogene amplified cancers. Their ability to target a central cancer growth pathway might put them in direct competition with BBI-355, depending on its specific mechanism.
Monoclonal Antibodies and ADCs
- Novel HER2-targeted Therapies: Despite the availability of HER2-targeted therapies, new monoclonal antibodies and ADCs with improved efficacy and reduced toxicity are in development. These include agents designed to deliver cytotoxic drugs directly to cancer cells, minimizing systemic toxicity and potentially offering superior safety profiles compared to existing HER2 therapies and possibly competing with BBI-355.
- EGFR-targeted Antibody Therapies: For cancers with EGFR amplification, new monoclonal antibodies that more effectively block EGFR signaling or induce immune-mediated cell death are underway. These could offer better efficacy or tolerability compared to current EGFR inhibitors.
Cell Therapy Strategies
- CAR-T Cell Therapies: Although primarily used in hematological malignancies, there is growing interest in developing chimeric antigen receptor (CAR) T-cell therapies for solid tumors with specific oncogenic drivers. If successful, CAR-T approaches could provide a revolutionary treatment model for oncogene amplified cancers, representing a novel class of competition for BBI-355.
Gene Therapy and CRISPR/Cas9-based Strategies
- Gene Editing: Advances in CRISPR/Cas9 technology offer the potential for directly correcting oncogenic mutations in patients' DNA. Though still in early stages, these approaches could provide curative treatments, significantly impacting the competitive landscape for drugs targeting the consequences of oncogene amplifications.
Treating oncogene amplified cancers has considerably evolved, with targeted therapies leading the charge. These treatments have significantly improved patient outcomes by precisely targeting the cancer cells while sparing normal cells, thereby reducing side effects compared to traditional chemotherapy. Here are some notable drugs, including recently approved branded ones, used to treat various oncogene amplified cancers:
HER2-Positive Breast Cancer
- Trastuzumab (Herceptin): A groundbreaking monoclonal antibody that targets the HER2 protein. It was one of the first HER2-targeted therapies and has dramatically improved outcomes for patients with HER2-positive breast cancer.
- Pertuzumab (Perjeta): Approved for use in combination with trastuzumab and chemotherapy, Pertuzumab is another monoclonal antibody that works by binding to a different epitope on the HER2 receptor.
- Ado-trastuzumab emtansine (Kadcyla): An antibody-drug conjugate (ADC) that combines Herceptin with a cytotoxic agent, specifically targeting HER2-positive cancer cells and minimizing exposure to the rest of the body.
- Trastuzumab deruxtecan (Enhertu): A newer ADC, it was granted approval for unresectable or metastatic HER2-positive breast cancer. Its high efficacy, even in pretreated patients, marks it as a significant advancement.
EGFR-Mutant Non-Small Cell Lung Cancer (NSCLC)
- Gefitinib (Iressa) and Erlotinib (Tarceva): Among the first EGFR tyrosine kinase inhibitors (TKIs) approved for NSCLC with specific EGFR mutations.
- Osimertinib (Tagrisso): A third-generation EGFR inhibitor designed to target T790M mutation, a common resistance mechanism to first-generation TKIs. Osimertinib has become a preferred first-line treatment due to its high efficacy and ability to cross the blood-brain barrier.
ALK-Positive NSCLC
- Crizotinib (Xalkori): A first-in-class ALK inhibitor that transformed the treatment landscape for ALK-positive NSCLC by targeting the ALK gene fusion.
- Alectinib (Alecensa) and Brigatinib (Alunbrig): Second-generation ALK inhibitors offering improved efficacy and reduced toxicity. They are preferred in treating ALK-positive NSCLC, especially for patients with CNS involvement.
BCR-ABL Positive Chronic Myeloid Leukemia (CML)
- Imatinib (Gleevec): A pioneer in targeted cancer therapy, Imatinib targets the BCR-ABL tyrosine kinase, a product of the Philadelphia chromosome translocation. It has dramatically improved the prognosis for CML patients.
- Dasatinib (Sprycel) and Nilotinib (Tasigna): Second-generation inhibitors that are more potent and can overcome some forms of resistance to Imatinib.
BRAF V600E Mutant Melanoma
- Vemurafenib (Zelboraf) and Dabrafenib (Tafinlar): Targeted BRAF inhibitors for treating metastatic melanoma with BRAF V600E mutations. They are often used in combination with MEK inhibitors (such as Trametinib) for enhanced efficacy.
Recently Approved Drugs
- Selpercatinib (Retevmo): Approved in 2020 for lung and thyroid cancers with RET mutations.
- Pralsetinib (Gavreto): Another RET inhibitor approved for RET fusion-positive NSCLC, underlining the expansion of targeted therapies towards less common genomic alterations.
The advent of these targeted therapies underscores a paradigm shift in the treatment of oncogene-amplified cancers. By focusing on the genetic underpinnings of the disease, these drugs offer a more personalized and often more effective approach to cancer treatment. Their development continues to evolve with the discovery of new targets and resistance mechanisms, promising a future of more tailored and effective cancer therapies.
BBI-825
Scientific background
RNR (Ribonucleotide Reductase) inhibitors present a compelling therapeutic rationale in the context of MAPK (Mitogen-Activated Protein Kinase) pathway-activated cancers through their multifaceted approach in targeting cell proliferation and DNA repair mechanisms. The MAPK pathway plays a crucial role in cell cycle regulation, signaling cells to proliferate, differentiate, or survive. In numerous cancers, this pathway is aberrantly activated, leading to uncontrolled cell growth and tumor progression.
RNR is pivotal in DNA synthesis and repair as it catalyzes the conversion of ribonucleotides into deoxyribonucleotides, the building blocks of DNA. By inhibiting RNR, these inhibitors effectively limit the pool of deoxyribonucleotides available for DNA synthesis and repair. This is particularly impactful in rapidly dividing cancer cells, which require a significant amount of these building blocks to sustain growth and proliferation. The scarcity of deoxyribonucleotides triggers a halt in DNA synthesis, leading to cell cycle arrest and ultimately cell death, thus providing a direct countermeasure to the tumor-promoting effects of MAPK pathway activation.
Moreover, cancer cells with activated MAPK pathways may exhibit an increased reliance on DNA repair mechanisms due to an elevated rate of DNA damage resulting from rapid cell division. This makes them particularly susceptible to the effects of RNR inhibitors. By undermining the DNA repair capacity of these cells, RNR inhibitors can enhance the cytotoxic stress, leading to a synthetic lethality in the context of MAPK pathway activation.
Therefore, the therapeutic rationale for employing RNR inhibitors in MAPK pathway-activated cancers hinges on their ability to exploit the inherent vulnerabilities of these cancer cells — namely, their excessive reliance on DNA synthesis and repair processes for survival and proliferation. This targeted approach not only promises to impede tumor growth effectively but also offers a strategy to circumvent the resistance often encountered with other therapeutic agents targeting the MAPK pathway directly.
The science underpinning the therapeutic use of Ribonucleotide Reductase (RNR) inhibitors in MAPK pathway-activated cancers is well-established in certain aspects, yet it still encompasses areas of ongoing research, debate, and emerging findings. Here's a breakdown of the certainty and debate surrounding these points:
- Role of MAPK Pathway in Cancer: The significance of the MAPK pathway in cancer cell proliferation, survival, and differentiation is well-documented, with extensive evidence indicating its aberrant activation in a variety of cancers. This provides a solid foundation for targeting this pathway for cancer therapy.
- Mechanism of Action of RNR Inhibitors: The basic biochemistry of RNR inhibitors affecting DNA synthesis by limiting the availability of deoxyribonucleotides is well understood. The mechanism by which these inhibitors halt DNA synthesis and repair, leading to cell cycle arrest and apoptosis in rapidly dividing cells, is supported by robust empirical evidence.
- Vulnerability of MAPK-Activated Cancers to RNR Inhibition: The concept that cancers with activated MAPK pathways might have an increased dependency on DNA repair mechanisms, making them more susceptible to the effects of RNR inhibitors, is supported by preclinical models and laboratory findings. However, translating these observations to clinical practice involves complexities that are currently the subject of active research and clinical trials. The exact extent of increased vulnerability and its implications for treatment strategies across different cancer types remains an area of exploration.
- Clinical Efficacy and Resistance: Although preclinical studies provide supportive evidence for the potential of RNR inhibitors in treating MAPK pathway-activated cancers, the clinical efficacy, optimal dosing, resistance mechanisms, and side-effect profiles are still being elucidated through clinical trials. The therapeutic window and the ability to selectively target cancer cells without causing undue harm to normal cells are also critical points of ongoing investigation.
- Combination Therapies and Synthetic Lethality: The strategy of using RNR inhibitors in combination with other therapies to induce synthetic lethality, especially in cancer cells with activated MAPK pathways, shows promise but is subject to research. The understanding of how best to combine these treatments to maximize efficacy while minimizing side effects is an evolving field.
In summary, while the foundational science of targeting MAPK pathway-activated cancers with RNR inhibitors is established, translating this knowledge into effective, clinically approved therapies involves uncertainties that are currently being addressed through continuous research and clinical validation. The overall level of evidence supporting the therapeutic rationale is strong, particularly in the basic understanding of RNR's role and the MAPK pathway's involvement in cancer. However, the clinical application, including the specifics of tumor response, resistance mechanisms, and patient outcomes, still requires further evidence for widespread adoption.
Specific literature directly linking Ribonucleotide Reductase (RNR) inhibitors to the treatment of MAPK pathway-activated cancers is niche and emerging. The precise interplay between RNR activity and MAPK pathway activation in cancer biology is complex and involves multiple cellular processes including DNA replication, repair, and cellular proliferation signaling. Here are generalized insights into supporting studies and conceptual frameworks:
- RNR Inhibition and Cancer: The rationale for targeting RNR in cancer therapy is well established, with numerous studies demonstrating that RNR inhibitors, such as hydroxyurea, can impede DNA synthesis in rapidly dividing cells, leading to cell cycle arrest. Evidence for the specific role of RNR in MAPK pathway-activated cancers, however, would extrapolate from the broader understanding of RNR's critical function in DNA synthesis and repair—processes that are often upregulated in cancer cells due to signaling pathway dysregulation such as that observed with MAPK activation.
- MAPK Pathway Activation in Cancer: The literature robustly supports the role of the MAPK pathway in various cancers, showing that its aberrant activation promotes oncogenesis, tumor growth, and survival through mechanisms like increased cell division and evasion of apoptosis. Key publications have detailed the molecular biology of MAPK pathway components, such as BRAF, KRAS, and ERK, and their contributions to malignancies including melanoma, colorectal cancer, and non-small cell lung cancer.
- Potential Linkages Between RNR and MAPK Pathway: While direct studies might be sparse, the potential linkage comes from the intersection of RNR's role in DNA synthesis and the MAPK pathway's role in cell proliferation. The MAPK pathway, once activated, can increase the metabolic and divisional demands of a cancer cell, indirectly necessitating the DNA synthesis machinery in which RNR plays a crucial role. Therefore, inhibiting RNR could strategically undermine the increased proliferation rate driven by MAPK pathway activation.
- Preclinical Studies and Insights: Preclinical models have provided insights into how RNR inhibitors could be beneficial in treating cancers with activated MAPK pathways. For instance, studies on hydroxyurea or newer RNR inhibitors may show efficacy in tumor models that are genetically modified to have activated MAPK signaling. Such studies help to build the theoretical foundation for clinical exploration, even if they do not represent direct evidence of clinical efficacy.
In summary, while direct, specific literature on RNR's role in MAPK pathway-activated cancers might be developing, the interconnected roles of RNR in DNA synthesis and repair and the MAPK pathway in cell proliferation and survival provide a conceptual rationale for targeting RNR in these contexts. As research progresses, more specific studies are likely to emerge, offering clearer insight into this therapeutic strategy's effectiveness and mechanistic underpinnings.
The therapeutic rationale for using Ribonucleotide Reductase (RNR) inhibitors in MAPK pathway-activated cancers rests on well-established biological principles, yet factoring in real-world clinical settings introduces both strengths and weaknesses to the evidence base. Here's a breakdown of these aspects:
Strengths of the Evidence Base
- Well-characterized Biochemical Mechanisms: The underlying biochemistry of RNR's role in DNA synthesis is thoroughly understood. This provides a strong mechanistic foundation, as inhibiting RNR directly impacts the DNA replication necessary for cancer cell proliferation.
- Preclinical Models: Preclinical studies have shown that targeting RNR can be effective in slowing down cancer growth, especially in rapidly proliferating cells, which rely heavily on DNA synthesis. These studies offer proof of concept for the therapeutic strategy.
- Multiplicity of Targets within Cancers: Cancers with activated MAPK pathways often exhibit aggressive growth and resistance to conventional therapies. RNR inhibitors offer a novel target, potentially circumventing some resistance mechanisms, especially in cancers where MAPK pathway activation drives malignancy.
- Evidence of MAPK Pathway's Role in Cancer: The critical role of the MAPK pathway in various cancers is well-documented, with evidence linking pathway activation to poor prognoses. This supports the rationale for intervening in this pathway, indirectly through RNR inhibition, as a viable therapeutic strategy.
Weaknesses of the Evidence Base
- Translational Gap: While biochemical mechanisms and preclinical findings are promising, there remains a significant translational gap to clinical efficacy. Many treatments that show promise in a lab setting fail to demonstrate significant benefits in clinical trials due to complexities such as off-target effects, toxicity, and heterogeneous tumor responses.
- Lack of Specific Clinical Data: As of the last update, there is limited specific clinical trial data on RNR inhibitors for MAPK pathway-activated cancers. Without robust clinical trial results, the true therapeutic potential remains speculative, based more on theoretical rationale than concrete evidence.
- Resistance and Adaptive Mechanisms: Cancers can develop resistance to therapies, including RNR inhibitors, through various mechanisms. The evidence base might not fully account for the adaptability of cancer cells and the potential for resistance to emerge, which can significantly impact the long-term efficacy of RNR inhibitors.
- Potential Toxicity and Side Effects: Inhibiting RNR affects a fundamental cellular process, posing a risk for toxicity and adverse effects in normal cells. The strength of the evidence might not yet fully elucidate the balance between therapeutic benefits and potential harm, especially with systemic administration of RNR inhibitors.
- Heterogeneity of Cancers: The variability and genetic diversity within and among cancer types challenge the one-size-fits-all approach. The evidence supporting RNR inhibitors in MAPK pathway-activated cancers may not account for the full spectrum of genetic, environmental, and lifestyle factors affecting treatment outcomes.
In conclusion, while the conceptual and mechanistic rationale for using RNR inhibitors in MAPK pathway-activated cancers is compelling, translating this into effective, safe, and reliable therapies requires overcoming significant hurdles. Addressing the weaknesses in the evidence base, particularly through targeted clinical trials and research, is essential for moving forward.
Clinical trial overview
Summary of Study Design
The study of BBI-825, a ribonucleotide Reductase (RNR) inhibitor, is a first-in-human, open-label, non-randomized, Phase 1/2 trial. It aims to assess the safety, determine the maximum tolerated dose (MTD), and recommend a Phase 2 dose (RP2D) for BBI-825 when administered as a single agent and in combination with selected targeted therapies. The study focuses on patients with locally advanced or metastatic non-resectable solid tumors that have progressed despite all standard therapies or for whom no standard or clinically acceptable therapies are available. The enrollment estimate is 42 subjects, with an anticipated start in March 2024 and estimated primary completion by February 2027.
The interventional model involves sequential assignment: initially testing BBI-825 as a single agent in dose escalation followed by combination therapy. It is designed as an open-label study without any masking, meaning both the researchers and participants know about the administered drug. The primary outcome measures include the frequency and severity of treatment-emergent adverse events (TEAEs) and the identification of the MTD/RP2D of BBI-825. Secondary outcome measures focus on pharmacokinetics like maximum observed plasma concentration (Cmax), trough observed plasma concentration (Ctrough), time to Cmax (Tmax), area under the concentration-time curve (AUC), and anti-tumor activity measured by RECISTv1.1 criteria.
Critiques of Study Design
- Open-Label Design: The lack of blinding could introduce bias, as knowing the treatment might influence participants' reporting of symptoms or outcomes. However, for early-phase trials focusing on safety and dosage, open-label designs are common.
- Sequential Assignment: While beneficial for early phase exploration, the sequential method of first evaluating the drug as a single agent before combination therapy could delay understanding the full potential of BBI-825 in combination treatments.
- Specific Population: The study's focus on patients with no further standard or clinically acceptable therapies underscores its potential for essential findings. However, it may also limit the generalizability of results to all patients with the specified solid tumors.
- Small Sample Size: A sample size of 42 participants might not be sufficient to detect all potential adverse events, especially rare ones, or fully assess efficacy signals.
Operational or Technical Challenges
- Recruitment: Identifying and enrolling patients who match the specific inclusion criteria (advanced solid tumors with no other treatment options) could be challenging and may slow down the study commencement.
- Adherence: Ensuring adherence to the twice-daily oral administration of BBI-825 could be challenging, especially over extended periods. This could affect the consistency of dose delivery and the interpretation of pharmacokinetic data.
- Safety Monitoring: Given the potent nature of the investigational drug and its novel target, rigorous and continuous monitoring for adverse events is required, potentially increasing the operational complexity and cost of the study.
- Pharmacokinetics Analysis: The comprehensive pharmacokinetics objectives, including Cmax, Ctrough, Tmax, and AUC, alongside the primary and secondary efficacy endpoints, demand thorough and timely sample collection and analysis, adding to the operational burden.
- Endpoint Validation: Measuring the anti-tumor activity using RECISTv1.1 in such a small and diverse patient population may be challenging. Variability in tumor types and stages could affect the consistency and interpretability of response assessments.
This design represents a thoughtful initial approach to determining the clinical utility of BBI-825 in a challenging patient population with significant unmet medical needs. Nonetheless, as with any early-phase clinical trial, iterative refinements based on emerging data will be crucial to optimize the study's design and operational execution.
The study design and eligibility criteria for the investigation of BBI-825 in patients with locally advanced or metastatic non-resectable solid tumors, specifically those with MAPK pathway activated cancers, appear to be thoughtfully constructed to both enable a clear determination of the drug's safety and efficacy, and to provide initial proof-of-concept evidence for its use in this specific population. Herein, a detailed examination of the study's appropriateness, focusing on primary and secondary endpoints, inclusion and exclusion criteria, and potential challenges related to reproducibility:
Appropriateness of Primary and Secondary Endpoints
- Primary Endpoints (Safety, MTD/RP2D): These are appropriate for a Phase 1 study, where the focus is on evaluating safety and determining the appropriate dosage levels for subsequent studies. These endpoints are crucial for first-in-human trials and are adequately designed to assess the feasibility of BBI-825 treatment in future phases.
- Secondary Endpoints (Pharmacokinetics and Anti-tumor Activity): The chosen pharmacokinetic parameters and the assessment of anti-tumor activity using RECISTv1.1 criteria are pertinent for evaluating the efficacy potential of BBI-825. These endpoints will provide essential insights into the drug's behavior in the body and its impact on tumor size, supporting proof-of-concept for its therapeutic use.
Inclusion / Exclusion Criteria
- Inclusion Criteria Focus: By specifying participants with locally advanced or metastatic non-resectable solid tumors that are refractory to standard therapies, the study ensures a target population that could notably benefit from novel treatments. The requirement for measurable disease as per RECIST Version 1.1 and adequate organ function are standard and appropriate, ensuring that participants can safely undergo treatment and their responses can be accurately measured.
- Exclusion Criteria Considerations: Excluding participants who have had prior exposure to selective RNR inhibitors is logical to avoid biased efficacy or safety profiles. Likewise, the exclusion of individuals with certain medical conditions or those taking strong enzyme inhibitors or inducers is prudent to mitigate potential confounding factors or drug interactions.
Potential Reproducibility Challenges
- Highly Specific Population: The strict inclusion and exclusion criteria, while necessary for safety and clarity of the drug's effects, might limit the generalizability of the study findings. The results might not be easily reproducible in a broader population, including those with different types or stages of cancer or concurrent health issues.
- Requirement for Tumor Tissue: The necessity for FFPE (Formalin-Fixed Paraffin-Embedded) tumor tissue could limit participation to patients who have accessible tumor samples, potentially introducing selection bias and affecting reproducibility in broader patient populations without such available specimens.
- MAPK Pathway Activation Specificity: Although not explicitly stated in the provided criteria, the study's focus on MAPK pathway activated cancers likely requires molecular or genetic testing to confirm pathway activation. This specificity, while critical for targeted drug development, may introduce challenges in consistently identifying eligible participants across different sites, potentially affecting the study's reproducibility and scalability.
To mitigate these challenges, subsequent studies could consider broadening eligibility criteria where possible and ensuring diverse patient recruitment. Additionally, incorporating biomarker or molecular profiling as part of the study design might enhance the understanding of the drug's action and ensure more targeted and effective treatment for patients with MAPK pathway activated cancers.
Market overview
MAPK pathway activated cancers
The Mitogen-Activated Protein Kinase (MAPK) pathway is a critical signaling cascade that plays a fundamental role in the regulation of cell growth, division, differentiation, and survival across various cell types. When functioning normally, this pathway helps to control the processes that dictate cell proliferation and death, ensuring the maintenance of cellular homeostasis and organismal health. However, aberrations in the MAPK pathway, often stemming from genetic mutations or alterations in pathway components, have been implicated in the development and progression of numerous cancers.
Pathology of MAPK Pathway Activated Cancers
Cancers with activated MAPK pathways typically present with dysregulated cell signaling that leads to unchecked cell proliferation and survival, promoting tumor growth and disease progression. Common mutations affecting the MAPK pathway involve components such as BRAF, RAS (KRAS, NRAS, HRAS), and MEK1/2. For instance, mutations in the BRAF gene (notably the V600E mutation) are prevalent in melanomas, non-small cell lung carcinoma, and thyroid cancers, among others.
Symptoms
- Melanomas may present as changing moles or skin lesions.
- Non-small cell lung carcinomas might manifest through cough, chest pain, and dyspnea.
- Thyroid cancers could lead to a palpable neck mass, difficulty swallowing, or changes in voice.
Prognosis
The prognosis of MAPK pathway-activated cancers varies significantly depending on the cancer type, stage at diagnosis, patient’s overall health, and response to treatment. Generally, early detection and targeted therapy against components of the MAPK pathway can improve outcomes. The development of inhibitors targeting BRAF, MEK, and ERK, for example, has significantly advanced treatment for melanomas and other cancers harboring specific mutations in the MAPK pathway.
Current Treatments
Treatment strategies for MAPK pathway-activated cancers may include surgery, radiation, chemotherapy, and targeted therapies. Targeted therapies, particularly, have shown promise in improving prognosis and quality of life for patients with these mutations. For instance, BRAF inhibitors (such as vemurafenib and dabrafenib) and MEK inhibitors (such as trametinib and cobimetinib) have been used successfully, especially in melanomas with BRAF V600E mutations.
Conclusion
MAPK pathway-activated cancers represent a complex and diverse group of malignancies that benefit from a growing understanding of their molecular underpinnings. Continuous research is crucial for identifying novel therapeutic targets within this pathway and for developing more effective, personalized treatment strategies that can better manage or potentially cure these challenging diseases. The development of BBI-825, a novel inhibitor targeting the MAPK pathway, represents a significant market opportunity in the field of oncology, particularly for the treatment of MAPK pathway-activated cancers. This pathway's critical role in cell proliferation and survival, coupled with its frequent dysregulation in various cancers, sets the stage for a targeted therapeutic approach. By focusing on cancers with specific mutations in the MAPK pathway, BBI-825 could offer a more personalized and effective treatment option for patients, addressing a key unmet medical need.
Reference to Other Successful Drugs
- BRAF Inhibitors such as vemurafenib (Zelboraf) and dabrafenib (Tafinlar) have shown remarkable clinical benefits in melanoma patients with BRAF V600E mutations.
- MEK Inhibitors, including trametinib (Mekinist) and cobimetinib (Cotellic), are used alone or in combination with BRAF inhibitors to enhance treatment efficacy and manage resistance.
- KRAS Inhibitors have emerged more recently, with sotorasib (Lumakras/Lumykras) being the first to receive FDA approval for the treatment of non-small cell lung cancer with specific KRAS mutations.
Market Opportunity
The market opportunity for BBI-825 lies in its potential to address several gaps in the current treatment landscape:
- Broad Applicability: Given the diverse range of cancers with MAPK pathway mutations, BBI-825 could potentially cater to a wide patient population, including those with melanoma, non-small cell lung cancer, and thyroid cancer, among others.
- Overcoming Resistance: The development of resistance to existing MAPK inhibitors is a pressing challenge. If BBI-825 can provide a viable option for patients who have developed resistance to current therapies, it would significantly expand its market potential.
- Improved Safety and Efficacy: If BBI-825 demonstrates a favorable safety profile and superior efficacy compared to existing treatments, it could become a preferred first-line therapy, especially for patients with specific genetic mutations in the MAPK pathway.
Unmet Medical Need
Despite advances, there remains an urgent need for therapies that can offer durable responses, overcome resistance mechanisms, and reduce adverse effects associated with current treatment options. Additionally, the identification and development of therapies for patients with less common MAPK pathway mutations still represent an area of unmet medical need.
Conclusion
Based on the success of other targeted therapies within this indication, BBI-825 has the potential to capture a significant market share, provided it demonstrates clear benefits in terms of efficacy, safety, and the ability to overcome or prevent resistance. The development and commercial success of BBI-825 will hinge on its differentiation from existing therapies, its applicability to a broad or previously underserved patient population, and its ability to address the ongoing challenges in treating MAPK pathway-activated cancers.Given the pivotal role of the Mitogen-Activated Protein Kinase (MAPK) pathway in various cancers and the development of resistance to first-line treatments, there has been significant research into novel therapies that target this pathway. These therapies, aimed at providing effective treatment options for MAPK pathway-activated cancers, could potentially compete with BBI-825 in the oncology market. Below are some of the promising treatment avenues and mechanisms that are being explored:
1. Next-Generation MAPK Inhibitors
While BRAF and MEK inhibitors have been successful, the emergence of resistance has led to the development of next-generation inhibitors with improved profiles. These include:
- Non-V600 BRAF Inhibition: Identifying and inhibiting non-V600 mutants of BRAF represents a significant area of interest, expanding the scope beyond the V600E mutation to target a broader range of BRAF mutations.
- Pan-RAF Inhibitors: Designed to inhibit multiple RAF isoforms, these drugs target the upstream of the MAPK pathway, potentially offering a way to overcome or delay resistance mechanisms that arise with traditional BRAF inhibitors.
2. Combination Therapies
Combining MAPK pathway inhibitors with other targeted therapies or immunotherapies is a promising strategy to enhance efficacy and combat resistance. For example:
- MAPK Inhibitors + Immunotherapy: The rationale is that by inhibiting the MAPK pathway, tumor cells may become more susceptible to immune-mediated destruction. Early research suggests that combining MAPK inhibitors with PD-1/PD-L1 checkpoint inhibitors could synergize to improve patient outcomes.
- MAPK Inhibitors + CDK4/6 Inhibitors: This combination aims to tackle resistance by simultaneously inhibiting cell cycle progression alongside MAPK pathway signaling. Early-phase trials are exploring this combination in various cancers.
3. Targeting Downstream Components
Innovations in targeting downstream components of the MAPK pathway, such as ERK inhibitors, offer a way to interrupt the signaling cascade at a later stage, potentially circumventing some resistance mechanisms seen with BRAF and MEK inhibitors.
4. Precision Medicine Approaches
Advancements in genomics and molecular profiling are enabling the identification of patient-specific mutations within the MAPK pathway, leading to more personalized treatment approaches. This might include:
- Mutation-Specific Inhibitors: Drugs designed to target less common mutations within the MAPK pathway could fill significant treatment gaps and provide options for patients who currently have limited targeted therapies.
- Biomarker-Driven Therapy Selection: The use of comprehensive genomic or proteomic analyses to guide the selection of targeted therapies could enhance the efficacy and reduce the toxicity of treatment by ensuring the right patients are matched with the most appropriate therapy.
Competition Consideration
For BBI-825 to succeed amidst these emerging treatments, it will need to demonstrate distinct advantages, such as superior efficacy, an ability to overcome or prevent resistance, a favorable safety profile, or applicability to a broader range of MAPK pathway-activated cancers. The competitive landscape will also be shaped by the speed of clinical development, regulatory approvals, and the ability to secure a place in treatment guidelines and protocols.
In conclusion, while BBI-825 has potential, its success will depend on differentiating itself within a rapidly evolving oncology market that includes a diverse range of emerging therapies targeting the MAPK pathway. Continuous innovation and an understanding of the complex dynamics of cancer signaling pathways will be key to developing successful treatments for MAPK pathway-activated cancers.
Boundless Bio is adopting a highly innovative approach to cancer treatment that centers on exploiting the distinct vulnerabilities of extrachromosomal DNA (ecDNA) in cancer cells. The foundation of their scientific strategy involves a comprehensive understanding and manipulation of ecDNA to disrupt cancer cell growth and overcome therapeutic resistance.
Overview of Scientific Strategy
- Spyglass Platform: This is a multifaceted system comprising:
- A curated library of oncogene-amplified cancer model systems, including ecDNA-enabled models and control models (ecDNA compendium or ecDC).
- A suite of analytical tools for detecting, quantifying, characterizing, monitoring, and perturbing ecDNA.
- Large clinical and preclinical bioinformatics databases to support insights into ecDNA prevalence, patient populations, clinical outcomes, and model library expansion.
- Target Identification and Validation: They have identified a range of potential targets that affect various steps in the ecDNA lifecycle, including segregation, replication, transcription, and repair. These targets are at different stages of validation and drug discovery.
- ecDNA-Directed Therapeutic Candidates (ecDTx): The company is pioneering the development of novel, small molecules targeting biological pathways essential for ecDNA function in cancer cells. These molecules aim to intercept critical vulnerability nodes in ecDNA biology.
Through the Spyglass platform, Boundless Bio seeks to unravel the complex biology of ecDNA, identifying key targets and biological pathways that are crucial for the survival and proliferation of ecDNA-harboring cancer cells.
Similar Approaches
Boundless Bio’s focus on ecDNA as a therapeutic target is relatively unique but falls within the broader field of precision oncology and targeted therapies. Other companies, such as CRISPR Therapeutics and Intellia Therapeutics, also target genetic abnormalities in cancer, although through different mechanisms like gene editing. What sets Boundless Bio apart is its comprehensive focus on ecDNA and its lifecycle.
Risks and Pitfalls
- Complexity of ecDNA: ecDNA is a relatively new area of study, and its biological complexity may pose challenges in fully understanding and effectively targeting its functions.
- Drug Resistance: There is always a risk that cancers may develop resistance to ecDTx, necessitating continuous research and development of next-generation therapies.
- Target Identification Accuracy: The potential for "missed targets" or the mischaracterization of ecDNA's role in certain cancers could lead to inefficacies in treatment strategies.
- Translation from Models to Humans: Success in model systems does not always translate to success in human trials, posing a significant risk to the development pipeline.
- Regulatory and Safety Hurdles: As with all novel therapeutic approaches, navigating regulatory approvals and ensuring patient safety, especially with the manipulation of genetic material, could present significant challenges.
Conclusion
Boundless Bio’s scientific strategy is grounded in a deep understanding of ecDNA and its pivotal role in cancer biology. By leveraging a comprehensive platform for the identification and validation of ecDNA-centric targets, the company is at the forefront of developing novel cancer therapies. However, the pioneering nature of their approach implies navigating substantial scientific, regulatory, and clinical challenges to successfully bring these therapies to market.
Weekly analyses of biotech startups, generated by AI
Receive high quality, AI-generated analyses of biotech startups, public companies and scientific papers each week.