CARGO Therapeutics investment analysis
November 8, 2023
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. Financial and valuation analysis were done by our proprietary software with inputs provided by LLMs. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Overview
CARGO Therapeutics is a clinical-stage biotech company focused on advancing cell therapies for cancer. It aims to overcome the limitations of existing CAR T-cell therapies, such as short-lived effects, safety issues, and inconsistent supply. The company’s lead candidate, CRG-022, targets CD22 to combat B-cell malignancies and is currently in Phase 2 trials after showing promising results in Phase 1, including a 53% complete response rate and substantial durability of response up to 43 months.
CRG-022 has achieved a reliable production rate and turnaround time, important factors considering the complexity of CAR T-cell therapy manufacturing. In addition to CRG-022, CARGO is developing a pipeline using proprietary platforms like CD2 and STASH to enhance efficacy, persistence, and safety of CAR T-cell treatments. These platforms also aim to address tumor resistance and immune escape by targeting multiple antigens and improving cell persistence.
CARGO's expertise is further underlined by its founding team’s experience in CAR T-cell therapy development and commercialization. The company seeks to establish itself as a leader in cell therapy, leveraging its technology to expand its CAR T-cell products beyond LBCL to other hematologic malignancies and exploring the potential utility of their platforms in a broader range of cancer types.
The company priced its IPO on November 9, 2023 on the NASDAQ with ticker CGRX.
CARGO Therapeutics Pipeline Overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial | Description |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRG-022 | CAR-T | CD22 | R/R LBCL-post CD19 CAR-T | CD22 CAR-T | |||||||
| CRG-022 | CAR-T | CD22 | LBCL-CAR-T naive | CD22 CAR-T | |||||||
| CRG-022 | CAR-T | CD22 | Pediatric B-ALL | CD22 CAR-T | |||||||
| CRG-023 | CAR-T | CD19, CD20, CD22 | B-cell malignancies | Tri-specific CAR-T with CD2 co-stimulation |
Highlights and risks
Compelling Phase 1 data supports use in CD19 R/R LBCL and potentially other indications
Best-in-class potential due to innovations in addressing tumor resistance and immune escape
Lead program in potentially pivotal Phase 2 study
Platform for next-generation CAR-T including tri-specific CAR-T, CD2 costimulation and STASH for improving homogeneity of CAR-T with multiple genetic inserts
Strong cash balance to support portfolio development
Manufacturing and commercialization of CAR-T products has proven challenging and expensive
Phase 2 study is conducted with different manufacturing process than Phase 1; if FDA does not accept comparability data, approval could be delayed
While backed by compelling preliminary science, further data is needed to validate that CARGO's innovations result in more effective and durable CAR-T therapies
Current valuation prices in approval after Phase 2; if FDA does not allow the current Phase 2 to be a pivotal study, approval could be delayed
CAR-T is a highly competitive space with over 100 programs in clinical trials for blood cancers
Valuation
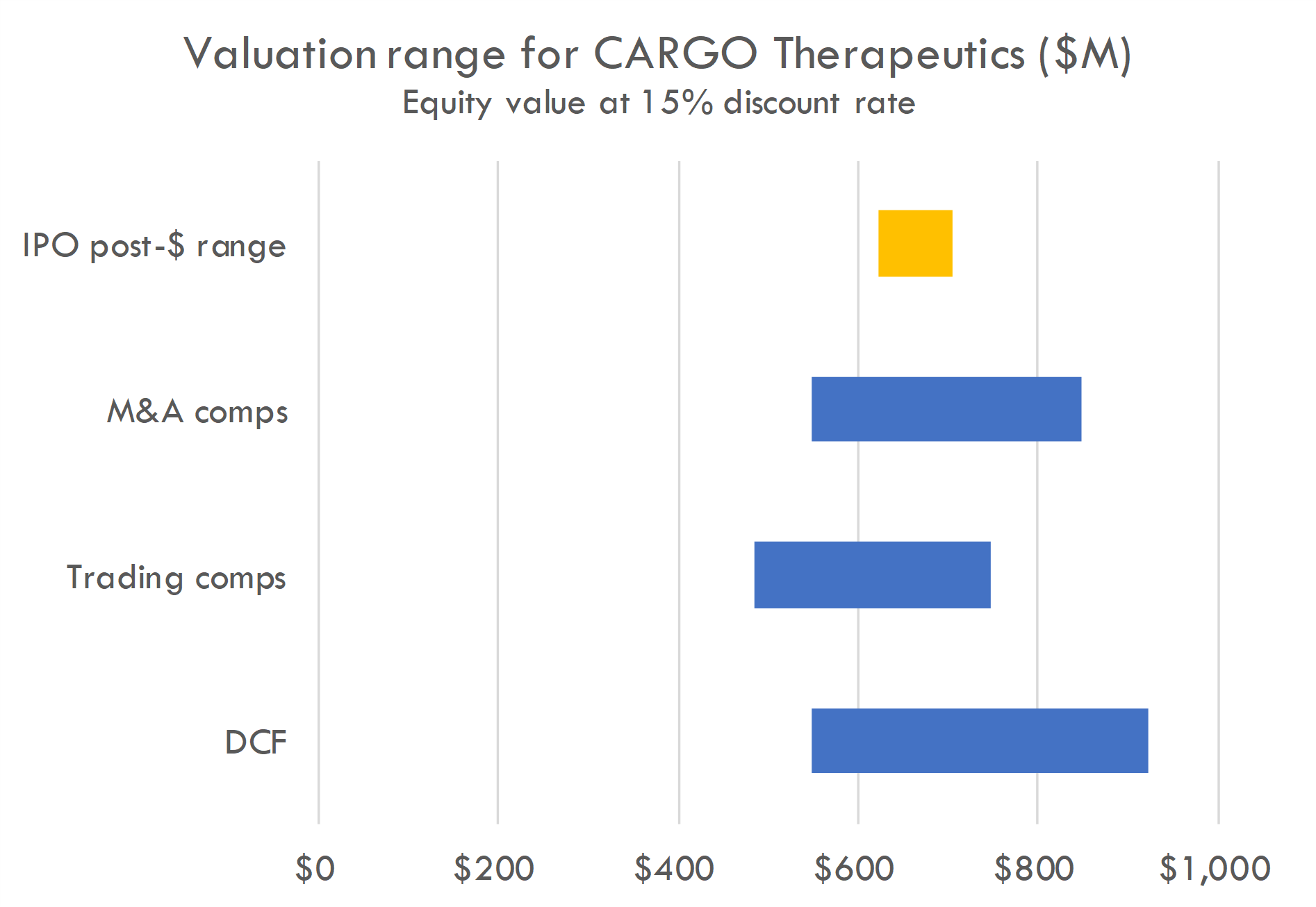
Our valuation analysis suggests a post-money equity value range of $600-800M using a 15% discount rate. This is in line with valuation implied by the proposed IPO pricing range.
The DCF is based on the revenue build below, a 55% probability of success in Phase 2 for CRG-022 in the CD19 R/R indication given positive Phase 1 data, and assumes the Phase 2 is a pivotal trial, with approval following successful Phase 2. We assume COGS as 30% of net revenue.
Trading comps include CRSP, NTLA, SRNEQ, SANA, AUTL, TSVT, GRCL, CRBU, ALLO, LYEL, ATRA, ADAP, FATE, DTIL, NKTX and ADCT.
M&A comps model acquisition after successful Phase 2 in two years, with probability of acquisition after Phase 2 ranging from 5-20%, and acquisition value ranging from $1 billion to $5 billion.
Funding history
CARGO Therapeutics' financing history includes:
- Convertible Notes: Issued $32 million in notes between April 2022 and January 2023, converted into Series A-2 Preferred Stock in February 2023.
- Series Seed Financing: In February 2021 and January 2022, they issued 810,700 shares of Series Seed Preferred Stock at $13.57 per share, raising a total of $11 million.
- Series A-1 Financing: In February 2023, they raised $68.8 million through the issuance of 5,072,919 Series A-1 Preferred Stock shares. Additional closings in July and October 2023 raised $131.9 million more, with shares priced at $13.57.
- Investors: Notable investors include Samsara BioCapital, Red Tree Venture Fund, and entities affiliated with Third Rock Ventures, among others.
Valuations were estimated at $31 million post-money for the Series Seed and $259 million post-money for the Series A-1 (the price per share was the same between the Series Seed and Series A-1), with respective pre-money valuations at $20 million and $57 million. Based on the midpoint of the IPO range of $16.00 per share, the IPO price is a 1.2x step-up to the Series A-1. This financing supports the company's clinical development and operational activities.
Analyze your company with our AI tools
Get an analysis like this one for your company or portfolio. Includes qualitative analysis and excel files with valuation analysis.
Contact us to learn more.
Addressing challenges in CAR-T development
CAR-T therapy represents a groundbreaking approach in cancer treatment, where T cells are genetically engineered to express chimeric antigen receptors (CARs) that can specifically target and eliminate tumor cells. This method has demonstrated potential curative outcomes in certain cancers, with FDA-approved therapies showing long-term benefits. Despite these successes, CAR-T faces significant challenges such as target-based and non-target-based resistance, immunogenicity of CAR constructs, and logistical hurdles in manufacturing and delivering these personalized treatments.
CARGO Therapeutics aims to address these limitations through several innovative strategies:
- Alternate Antigen Targeting: CARGO's lead candidate, CRG-022, targets CD22, a different antigen than CD19, which is commonly expressed in B-cell tumors. This could overcome resistance due to the loss or change in CD19 antigenicity.
- Multi-Antigen Specificity: Their preclinical candidate, CRG-023, is a tri-specific CAR-T therapy that targets CD19, CD20, and CD22, potentially preventing relapse due to antigen loss or modulation.
- CD2 Costimulation Platform: This technology counters the resistance mechanism caused by the loss of CD58 expression on tumor cells. By integrating a CD2 costimulatory domain, the CAR T cells can be activated even in the absence of CD58, enhancing the immune response against the tumor.
- Fully-Human Binders: CARGO utilizes human binders in their CAR constructs to reduce the potential immunogenicity of the therapy, which can lead to the body attacking the infused CAR T cells.
- STASH Platform: To address the manufacturing challenge of producing homogenous CAR-T cells with multiple genetic inserts, CARGO has developed the STASH technology which ensures only cells with the full set of desired genetic modifications are selected.
CARGO Therapeutics' approach addresses key resistance mechanisms in current CAR-T therapies. Their multi-antigen targeting strategy could significantly reduce the risk of relapse due to antigen loss, a common failure point in monospecific CARs. The CD2 costimulation platform is a clever solution to the problem of decreased T-cell activation due to the absence of CD58, which could improve the efficacy of the CAR-T cells. Additionally, using fully-human binders to construct their CARs could minimize the risk of immune rejection, potentially allowing for longer persistence of the CAR-T cells in the body.
The STASH platform seems to be a promising solution to the complexities of manufacturing CAR-T therapies with multiple genetic modifications, addressing a critical bottleneck in delivering these treatments to a broader patient population.
However, these innovations also face potential challenges. The clinical success of therapies targeting alternate antigens like CD22 still needs to be validated in larger and more diverse patient populations. While the CD2 costimulation technology seems promising in theory, its practical effectiveness will need to be demonstrated in clinical settings, and its impact on the broader immune system needs careful monitoring. Similarly, the human binder approach may reduce immunogenicity, but the immune responses in patients could still vary widely.
The STASH platform’s ability to create a homogeneous product from multiple vectors is innovative, yet the technology must prove scalable and reliable in a commercial setting. There's also the regulatory challenge, as the FDA must be convinced of the comparability of CARGO's manufacturing process to that used in earlier trials, which is not always straightforward.
First-gen CAR-T
CAR-T (Chimeric Antigen Receptor T-cell) therapies are a form of immunotherapy that involves reprogramming a patient's own immune cells to recognize and attack cancer cells. CAR-T therapies use a patient's T cells (a type of white blood cell) and modify them to produce special structures called chimeric antigen receptors (CARs) on their surface. These receptors are designed to bind to specific proteins on the surface of cancer cells.
The process for manufacturing CAR-T therapies is different from traditional therapies. First, T cells are collected from a patient’s blood in a process similar to blood donation. Then the collected T cells are then genetically engineered in a laboratory to express CARs that will target cancer cells. These modified cells are grown in the lab until they number in the millions or billions, then the expanded batch of CAR-T cells is infused back into the patient.
Once infused back into the patient, the engineered CAR-T cells seek out and bind to cancer cells through the specific antigens they were designed to target. Upon binding, they become activated and kill the cancer cells.
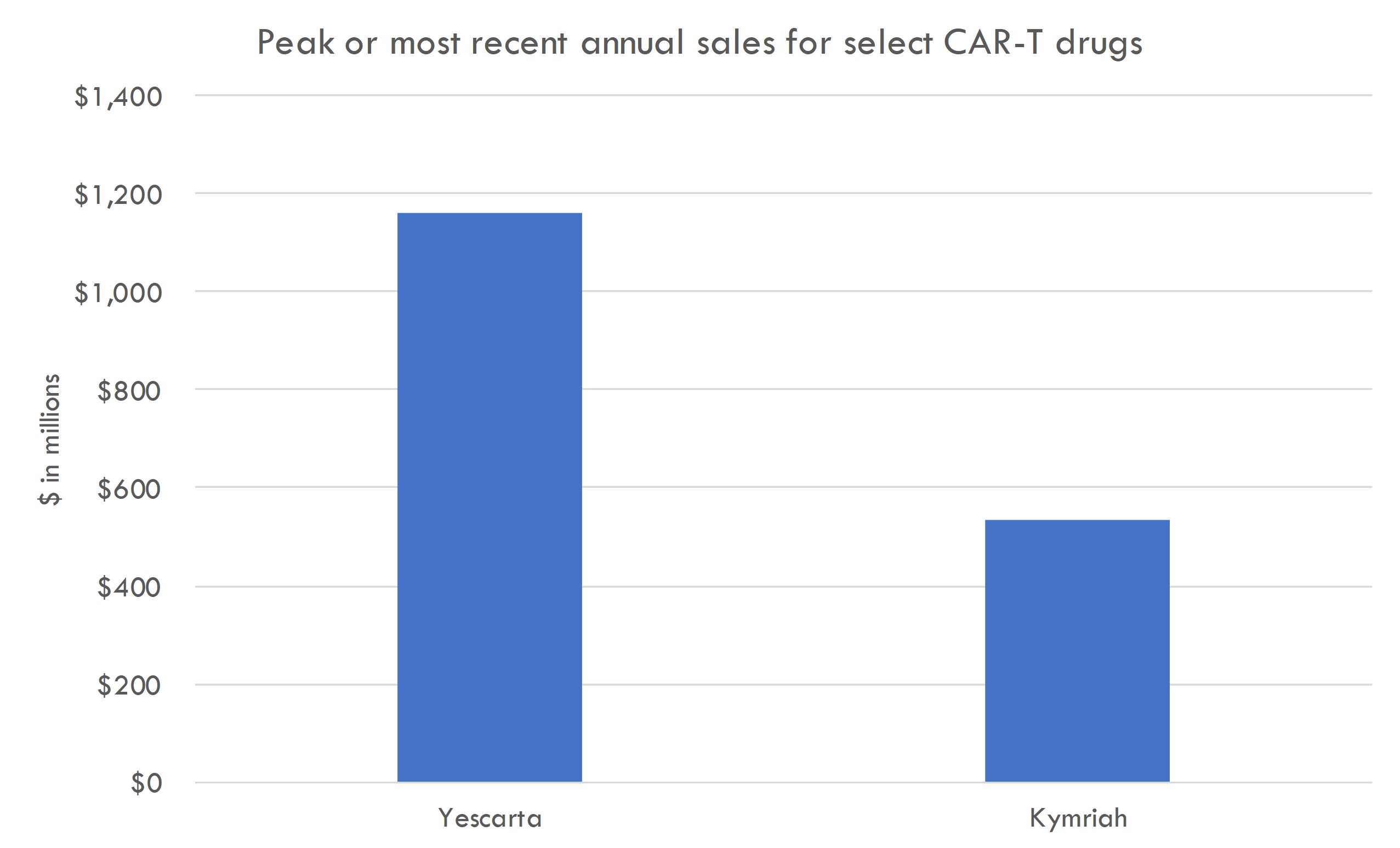
The first-generation CAR-T cell therapies, primarily targeting the CD19 antigen on B-cell leukemias and lymphomas, showed remarkable clinical efficacy in trials. For instance, therapies like tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) demonstrated high remission rates in patients with relapsed or refractory B-cell malignancies, leading to their FDA approval.
These therapies have been commercially available for several years, with sales growing as they treat more patients. However, they are expensive, with treatments costing hundreds of thousands of dollars, which has impacted their widespread adoption.
Challenges associated with these therapies include:
- Efficacy: Some patients relapse after treatment as their cancer develops resistance to the therapy.
- Safety: There can be severe side effects, including cytokine release syndrome (CRS) and neurotoxicity.
- Accessibility: Not all patients are eligible due to the aggressive nature of their disease or due to comorbidities.
- Manufacturing: The personalized nature of CAR-T therapy makes it complex and costly to produce.
- Cost: The high price point makes it inaccessible for many patients and burdensome for healthcare systems.
- Delivery: The treatment requires specialized centers and expertise, limiting availability.
CARGO's potential solutions
CARGO's approach aims to address several limitations of existing CAR-T therapies, including development of resistance, reducing immunogenicity, and improving the manufacturing process.
Resistance to therapy
The rate of relapse or resistance to CD19 CAR-T therapy can vary widely depending on several factors, including the type of hematologic cancer being treated, the specific product used, and patient characteristics.
For example, in the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), CD19 CAR-T therapies have shown high initial response rates, often greater than 80%. However, longer-term follow-up indicates that a significant proportion of patients may experience relapse within the first year following treatment. For these patients, the relapse rate can be up to 50% or more.
In the case of diffuse large B-cell lymphoma (DLBCL), another common indication for CD19 CAR-T therapy, initial response rates are also high, but approximately 30-50% of patients may relapse or have resistant disease after treatment, with many relapses occurring within the first few months post-infusion.
These rates are not static and are subject to change as longer-term follow-up data becomes available and as new treatment regimens and second-generation CAR-T therapies are developed. It's also important to note that ongoing clinical trials and real-world data continue to provide more insights into the durability of these therapies and the patterns of resistance or relapse.
Resistance to CD19-targeted CAR-T cell therapy in patients with B-cell malignancies can occur through several mechanisms:
- Antigen Escape
- Loss of Target Antigen (CD19): The most common mechanism of resistance is the loss or downregulation of the CD19 antigen on the surface of cancerous B cells. This can occur through gene mutations, alternative splicing, or lineage switching where the cancerous cells differentiate into a type that does not express CD19.
- Antigen Masking: Tumor cells may mask the CD19 antigen with other molecules, preventing CAR-T cells from recognizing and binding to them.
- Modulation of the Tumor Microenvironment
- T-cell Exhaustion: Continuous exposure to antigens and inflammatory signals can lead to T-cell exhaustion, where T cells lose their ability to function effectively.
- Immunosuppressive Microenvironment: Tumors can create an immunosuppressive microenvironment that inhibits CAR-T cell function through the production of inhibitory cytokines, recruitment of regulatory T cells, or upregulation of immune checkpoint molecules.
- CAR-T Cell Intrinsic Factors
- Poor CAR-T Cell Persistence: In some cases, CAR-T cells may not persist for long enough in the body to exert a sustained antitumor effect.
- Inadequate CAR-T Cell Expansion: There may also be insufficient proliferation of CAR-T cells following infusion into the patient.
- Intrinsic Tumor Cell Resistance
- Genomic Instability of Tumor Cells: Tumor cells often have high rates of mutation, which can lead to the emergence of clones that are resistant to therapy.
- Heterogeneity of Antigen Expression: Not all tumor cells express CD19 at levels sufficient for CAR-T cell targeting, allowing some cells to evade detection and destruction.
- Relapse with CD19-Negative Disease
- Lineage Switching: Some B-cell malignancies can undergo lineage switching, transforming from a B-cell phenotype to a myeloid or other lineage that does not express CD19.
- Immunogenicity of the CAR Construct:
- Immune Response to CAR: The patient’s immune system may mount a response against the engineered CAR-T cells, leading to their elimination before they can effectively eradicate the tumor.
CARGO has several strategies for addressing resistance. Some are employed by lead program CRG-022, while others are used in earlier-stage programs.
Here is an overview:
- Targeting Alternate Antigens: CARGO's lead product, CRG-022, targets CD22, an antigen that is consistently expressed on B-cell tumors, including those resistant to CD19-targeted therapies. This could help overcome antigen escape mechanisms where tumors lose or mask the CD19 antigen.
- Fully-Human Binders: By using fully-human binders in their CAR constructs, CARGO aims to reduce the risk of immunogenicity against the CAR-T cells, potentially increasing their persistence and reducing the chances of rejection by the patient’s immune system.
- Addressing splice variants: CARGO is also addressing issues such as splice variants of target antigens, which can lead to resistance. Their use of an antibody known as m971 binds to the membrane-proximal domains of CD22, which may be less prone to Splice Variant-Related Resistance.
- Additionally, the design elements like the short linker in the scFv and the inclusion of the 4-1BB costimulatory domain are expected to enhance the efficacy and persistence of their CAR-T cells.
- Multi-specific CAR-T Cells: Their multi-specific CAR-T candidate, CRG-023, is designed to target multiple B-cell antigens (CD19, CD20, and CD22). This broadens the range of targetable cancer cells, potentially overcoming resistance due to loss or downregulation of a single antigen.
- CD2 Costimulatory Signaling: To address the issue of non-target-based resistance, such as loss of costimulatory ligands like CD58, CARGO is creating CAR-T cells that can induce CD2 costimulatory signaling regardless of CD58 expression. This could enhance CAR-T cell activation and function even when traditional costimulatory signals are diminished. This innovation is used by their preclinical programs.
The strategies employed by CARGO for their CRG-022 therapy are based on scientific principles derived from both preclinical and clinical experiences with CAR-T cell therapies. Let's examine the level of evidence supporting each of their approaches:
Targeting CD22 The rationale for targeting CD22 is supported by evidence that it is consistently expressed on the surface of B-cell malignancies. This makes it a viable target, especially for tumors that have lost or downregulated CD19, leading to resistance against CD19-targeted CAR-T therapies. Clinical trials with other CD22-targeted therapies, including inotuzumab ozogamicin, have demonstrated the effectiveness of targeting CD22 in B-cell malignancies.
Fully Human Binders The use of fully human scFv is intended to reduce the immunogenicity of the CAR construct. Clinical experiences with non-human scFvs (e.g., murine-derived) have shown that they can elicit an immune response, potentially leading to rejection or rapid clearance of CAR-T cells. Fully human scFvs are less likely to be recognized as foreign, which could improve the persistence and efficacy of CAR-T cells. This is supported by immunological principles and some clinical evidence, although direct comparative data between fully human and murine/human chimeric CAR-T cells are still emerging.
Binding to Membrane-Proximal Domains The hypothesis is that targeting membrane-proximal epitopes on antigens could enhance the activation and potency of CAR-T cells. This is based on the proximity of the CAR to the T-cell membrane, which may enhance signaling cascades essential for T-cell activation. Some preclinical studies support this idea, but conclusive clinical data are limited.
Shorter Linker in the scFv A shorter linker between the variable heavy and variable light chains in the scFv may lead to better structural stability and dimerization, which is hypothesized to enhance signaling and CAR-T cell activation. While there are preclinical studies that suggest shorter linkers might improve the function of CAR-T cells, translating these findings into improved clinical efficacy is an ongoing area of research.
Using a 4-1BB Costimulatory Domain The choice of costimulatory domains within CAR constructs can influence the phenotype, persistence, and efficacy of CAR-T cells. The 4-1BB domain has been associated with a more memory-like phenotype and longer persistence compared to other domains like CD28. This is well-supported by clinical data, as CAR-T therapies utilizing the 4-1BB domain, such as axicabtagene ciloleucel, have shown durable responses in patients.
As a whole, CARGO's scientific strategies for CRG-022 are grounded in established CAR-T cell therapy science, but as with all investigational therapies, clinical trials will be the definitive test of their efficacy and safety. The science supporting these ideas continues to evolve, and CARGO’s clinical results will contribute valuable data to this body of knowledge.
In addition to the innovations employed for CRG-022, CARGO's earlier stage programs utilize several other innovations:
- CD2 Costimulation Platform Technology: The concept of costimulation is well-established in immunology, and CD2 is a known costimulatory receptor. The specific hypothesis that CD2 signaling can compensate for CD58 downregulation is more novel and would be supported by preclinical evidence, such as cell-killing assays. While promising, clinical validation is necessary to establish its effectiveness in patients.
- Tri-Specific CAR-T: The idea of multi-specific targeting is supported by both preclinical and early clinical data indicating that targeting multiple antigens can overcome resistance due to loss or modulation of a single antigen. The broader the antigen spectrum, the less likely tumor cells can evade detection and destruction.
- STASH Technology: The STASH technology is innovative and addresses a known challenge in CAR-T cell manufacturing. The approach has a strong scientific rationale and preclinical backing. The purification of cells that have successfully incorporated all vectors is a clever solution to ensure product uniformity, which is critical for clinical effectiveness. However, the clinical success of this approach will depend on the demonstration of its scalability and reproducibility.
Improved manufacturing
The manufacturing process for CAR-T therapies is intricate and involves several critical steps:
- Apheresis: The process starts with collecting the patient’s T cells through apheresis, which can be a lengthy and complex process requiring specialized equipment.
- Activation: T cells must be activated before genetic modification, often using artificial antigen-presenting cells or specific antibodies.
- Transduction: The activated T cells are then genetically modified, typically using viral vectors, to express the CAR that will target the cancer cells.
- Expansion: After modification, the CAR-T cells are cultured and expanded to create a large enough population for therapeutic use.
- Formulation: The expanded CAR-T cells are then formulated into a product that can be infused back into the patient.
- Quality Control: Before release, the CAR-T cells undergo rigorous testing to ensure they meet established criteria for potency, purity, and safety.
- Cryopreservation: Finally, the cells are often cryopreserved for shipping and later infused into the patient after a conditioning chemotherapy regimen.
Challenges in the manufacturing process include:
- Starting Material Variability: The process begins with the patient's own T cells, which can vary in quality and quantity due to factors such as prior treatments, disease burden, and overall health.
- Complexity of Cell Engineering: Genetic modification of T cells requires specialized viral vectors, which are not only difficult to produce but also must be handled with care to ensure safety and efficacy.
- Scalability: CAR-T therapies are currently manufactured on a per-patient basis. Scaling up for treating large numbers of patients is challenging and requires a balance between customization and standardization.
- Manufacturing Time: The entire process, from T cell collection to reinfusion, can take several weeks—a significant period for patients with rapidly progressing diseases.
- Product Consistency: Ensuring that each batch of CAR-T cells is consistent in terms of potency and composition is difficult due to the nature of living cells as the product.
- Cost: The intricacies of CAR-T cell therapy manufacturing contribute to a high cost, which can be a barrier to access for many patients and healthcare systems.
- Quality Control: Each step of the process requires rigorous testing to ensure the product meets stringent safety and efficacy standards. Establishing robust release assays for CAR-T products, including tests for potency, purity, and identity, is challenging.
- Logistics: The "vein-to-vein" time, including shipping cells to and from manufacturing facilities, needs to be optimized to reduce the risk of cell viability loss and to provide timely treatment to patients.
- Regulatory Requirements: Complying with regulatory standards for advanced therapeutic medicinal products (ATMPs) can be demanding, as the regulatory landscape continues to evolve with the technology.
- Infrastructure: Specialized facilities are required for CAR-T cell manufacturing, which must be designed to comply with Good Manufacturing Practices (GMP) and ensure a sterile environment.
- Cryopreservation: Not all CAR-T cells respond well to the freeze-thaw process, which can impact their viability and function.
- Capacity: As the demand for CAR-T therapies grows, expanding manufacturing capacity while maintaining quality is a significant hurdle.
- Personnel: Trained personnel are essential for the complex CAR-T manufacturing process. There is a need for specialized scientists, quality control experts, and technicians.
- Handling and Administration: Once the product is ready, it requires specialized handling and administration, with trained medical staff and facilities equipped to manage potential side effects.
CARGO plans to address these issues through:
- Robust and Transferable Manufacturing Processes: CARGO intends to create manufacturing processes that are reliable and easily transferable to other facilities, including contract development manufacturing organizations (CDMOs), to expand capacity and reduce turnaround times. This approach can help address the scalability challenge, allowing the production of CAR-T cells for a larger patient population.
- Automation and Process Improvements: By automating certain steps, such as filling, and closing the process, CARGO aims to increase throughput, enhance reliability, and minimize costs. Automation can also help to standardize the manufacturing process, which contributes to the consistency and quality of the CAR-T cell products.
- Introduction of Process Design Features Pre-Pivotal Trial: Implementing key process and method changes before starting pivotal clinical trials could reduce the need for changes post-trial, streamlining the path to commercialization and regulatory approval.
- Cryopreservation of Apheresis Material: The ability to cryopreserve starting material enables more efficient use of manufacturing slots and provides a more flexible supply chain, potentially improving the availability of CAR-T therapies to patients in wider geographic areas.
- Securing Reliable Vector Supply: Collaboration with a CDMO for the consistent production of the lentiviral vector ensures a steady supply, addressing a critical component of the CAR-T manufacturing process.
- Cost of Goods: By focusing on automation and the supply of critical reagents, CARGO is working to reduce the cost of goods, which could make CAR-T therapies more accessible.
- Predictable Manufacturing Turn-Around-Time: CARGO believes that the process and operational improvements will provide greater control over manufacturing turnaround time, which is crucial for timely treatment of patients.
- Regulatory Strategy: Leveraging the experience from pioneering CAR-T products, CARGO is working to anticipate and manage regulatory challenges. Establishing a suitable potency assay and qualifying all release methods beforehand is an example of proactive regulatory strategy.
- Comparability Strategies: Utilizing current regulatory guidance, CARGO designs comparability strategies to manage changes throughout the product lifecycle, aiming to simplify later efforts to establish comparability across manufacturing sites.
- Expanding Manufacturing Network: By focusing on these manufacturing strategies prior to the Phase 2 clinical trial, CARGO plans to simplify the process of expanding their manufacturing network to meet demand rapidly.
Scientific and technical literature supports the feasibility of developing robust manufacturing processes. However, the transferability of these processes across different facilities, especially when involving third-party manufacturers (CDMOs), can be complex due to variations in equipment, personnel training, and local regulatory requirements.
Automation is a growing trend in biomanufacturing and can lead to more consistent products. The literature and industry case studies suggest that automation can significantly enhance reproducibility and efficiency. This goal seems quite achievable and aligns with current industry best practices.
Ensuring a reliable supply of viral vectors is challenging due to the complexities of vector production. The business landscape shows an increasing number of CDMOs specializing in vector production, which could help CARGO secure the needed supply. While this is a reasonable goal, it is contingent on maintaining strong supplier relationships and may require contingency plans for supply chain disruptions.
Reducing the cost of goods is critical for commercial success. While efforts to automate and optimize the manufacturing process can contribute to cost reduction, the inherently expensive nature of personalized therapy means that significant cost reductions may be more challenging to achieve.
Achieving a predictable manufacturing turnaround is challenging, given the complexity of CAR-T manufacturing and variability in starting materials. While process optimizations can lead to improvements, completely predictable turnaround times are difficult to guarantee.
Analyze your company with our AI tools
Get an analysis like this one for your company or portfolio. Includes qualitative analysis and excel files with valuation analysis.
Contact us to learn more.
Pipeline analysis
CRG-022
CRG-022 is an autologous chimeric antigen receptor (CAR) T-cell therapy developed by CARGO Therapeutics, designed to target CD22, a protein commonly expressed on B-cell malignancies. This therapeutic approach is aimed particularly at treating relapsed or refractory (R/R) large B-cell lymphoma (LBCL) patients who have become resistant to CD19-targeted CAR-T therapies
CRG-022 targets CD22, which is expressed in a high percentage of DLBCL patients, making it a promising alternative to CD19-targeted therapies, especially in cases where CD19 expression is lost. It incorporates a short linker in its single-chain variable fragment (scFv), which may increase dimerization and improve efficacy. The scFv targets a membrane-proximal epitope on CD22, aiming to minimize resistance that can arise from alternative splicing or other genetic alterations affecting the antigen. CRG-022 is composed entirely of human sequences, which helps in reducing the immunogenicity that could otherwise lead to the rejection of the therapy. The CAR construct includes a 4-1BB costimulatory domain, known to enhance the long-term persistence and survival of CAR-T cells in patients.
CRG-022 is being developed to treat LBCL patients who are resistant to CD19 CAR-T therapy, addressing an unmet need for patients with poor prognosis after standard therapies. Early clinical trials have shown promising results, with a significant number of patients achieving complete response (CR) and a median overall survival of 14.1 months. The treatment has been generally well-tolerated, with manageable adverse events primarily related to cytopenias (neutropenia, anemia, and thrombocytopenia), which are common to CAR-T therapies. It has demonstrated a favorable manufacturing success rate, with a median turnaround time of 18 days, which is critical in providing timely treatment to patients.
A multi-center Phase 2 clinical trial commenced in August 2023, with interim results expected in 2025.
Beyond R/R LBCL, CARGO is evaluating CRG-022 for use in CAR T-naïve LBCL patients, as well as in B-cell acute lymphoblastic leukemia (B-ALL), where a similar CD22-targeted CAR-T therapy has shown a 70% CR rate in a Phase 1 NCI trial.
Scientific thesis
CD22 is a promising target for CAR T-cell therapy in B-cell malignancies because it is widely expressed on both healthy and cancerous B cells. It is present in the majority of diffuse large B-cell lymphoma (DLBCL) and B-cell acute lymphoblastic leukemia (B-ALL) patients. Crucially, CD22 remains expressed even when CD19 is lost, which often occurs in patients who have developed resistance to CD19-targeted CAR T-cell therapies. Therefore, targeting CD22 could provide an effective treatment option for patients whose cancer has relapsed or is refractory to treatments that target CD19.
The design includes several key features:
- Membrane Proximal Binding: CRG-022 targets CD22 close to the cell membrane, which may enhance CAR T-cell activation due to the proximity to intracellular signaling domains. This approach aims to address the challenge of antigen variability, including splice variants that may result in resistance to therapy.
- Short Linker: The CAR uses a short linker in its scFv, which connects the antibody domains. Short linkers have been associated with increased dimerization of the CAR molecules, potentially improving their activation and efficacy.
- 4-1BB Costimulatory Domain: CRG-022 includes the 4-1BB costimulatory domain within its CAR construct, which is known to contribute to the long-term persistence and memory phenotype of CAR T-cells. This feature could lead to more durable responses and has been linked to better clinical outcomes and fewer serious adverse events in other models.
- Fully Human Antigen-Binding Domain: The therapy uses a fully human scFv to reduce the risk of immunogenicity, which can cause the patient's immune system to attack the CAR T-cells, leading to reduced persistence and increased risk of disease relapse. Fully human CAR constructs are believed to be less likely to provoke such immune responses compared to those containing murine sequences.
The strategies employed by CARGO for their CRG-022 therapy are based on scientific principles derived from both preclinical and clinical experiences with CAR-T cell therapies. Let's examine the level of evidence supporting each of their approaches:
Targeting CD22 The rationale for targeting CD22 is supported by evidence that it is consistently expressed on the surface of B-cell malignancies. This makes it a viable target, especially for tumors that have lost or downregulated CD19, leading to resistance against CD19-targeted CAR-T therapies. Clinical trials with other CD22-targeted therapies, including inotuzumab ozogamicin, have demonstrated the effectiveness of targeting CD22 in B-cell malignancies.
Fully Human Binders The use of fully human scFv is intended to reduce the immunogenicity of the CAR construct. Clinical experiences with non-human scFvs (e.g., murine-derived) have shown that they can elicit an immune response, potentially leading to rejection or rapid clearance of CAR-T cells. Fully human scFvs are less likely to be recognized as foreign, which could improve the persistence and efficacy of CAR-T cells. This is supported by immunological principles and some clinical evidence, although direct comparative data between fully human and murine/human chimeric CAR-T cells are still emerging.
Binding to Membrane-Proximal Domains The hypothesis is that targeting membrane-proximal epitopes on antigens could enhance the activation and potency of CAR-T cells. This is based on the proximity of the CAR to the T-cell membrane, which may enhance signaling cascades essential for T-cell activation. Some preclinical studies support this idea, but conclusive clinical data are limited.
Shorter Linker in the scFv A shorter linker between the variable heavy and variable light chains in the scFv may lead to better structural stability and dimerization, which is hypothesized to enhance signaling and CAR-T cell activation. While there are preclinical studies that suggest shorter linkers might improve the function of CAR-T cells, translating these findings into improved clinical efficacy is an ongoing area of research.
Using a 4-1BB Costimulatory Domain The choice of costimulatory domains within CAR constructs can influence the phenotype, persistence, and efficacy of CAR-T cells. The 4-1BB domain has been associated with a more memory-like phenotype and longer persistence compared to other domains like CD28. This is well-supported by clinical data, as CAR-T therapies utilizing the 4-1BB domain, such as axicabtagene ciloleucel, have shown durable responses in patients.
As a whole, CARGO's scientific strategies for CRG-022 are grounded in established CAR-T cell therapy science, but as with all investigational therapies, clinical trials will be the definitive test of their efficacy and safety. The science supporting these ideas continues to evolve, and CARGO’s clinical results will contribute valuable data to this body of knowledge.
Phase 1 study overview
CRG-022 has been studied in one Phase 1 study with two studies ongoing, and a potentially pivotal Phase 2 study began dosing in summer 2023.
The Phase 1 clinical studies of CRG-022, an autologous CAR T-cell therapy targeting CD22, involved adult patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) who had previously received CD19 CAR T-cell therapy
Here is a summary of the Phase 1 results:
- The trial enrolled 41 adult patients with R/R LBCL.
- A 95% manufacturing success rate was achieved, with 38 out of 40 patients receiving the therapy.
- The overall response rate (ORR) was 68%, with a complete response (CR) rate of 53%.
- Progression-free survival (PFS) at 6 months was 47%, with a median PFS of 3.0 months.
- Median survival for the overall patient population was 14.1 months.
- CRS was mostly mild to moderate, with only one patient experiencing Grade 3 CRS.
- Serious adverse events included sepsis/infection, cardiac disorders, and a second peak of CRS that led to hospitalizations.
- Common treatment-related adverse events were neutropenia, anemia, and thrombocytopenia.
The successful manufacturing rate and the median turnaround time indicate an efficient production process. The ORR and CR rates are promising, suggesting that CRG-022 has significant antitumor activity in a patient population with limited treatment options. The lower incidence of severe CRS and ICANS compared to some CD19-targeted CAR T-cell therapies may indicate a favorable safety profile.
The median PFS and overall survival, while encouraging, indicate that there is still a significant proportion of patients who do not achieve long-term remission. The incidence of HLH, a serious and potentially fatal side effect, particularly at the higher dose level, is a concern. The trial has observed serious adverse events, including deaths potentially related to the treatment, highlighting the need for careful patient selection and management.
The data suggests that CRG-022 has considerable antitumor activity in LBCL with similar ORR and CR rates across both dose levels, which may indicate that increasing the dose does not significantly enhance the efficacy. The ORR and CR rates are clinically significant given the difficult-to-treat nature of the disease in this patient population, who had previously received multiple lines of therapy including CD19 CAR-T. There is no clear dose-dependence for ORR or CR rate, which is beneficial from a safety perspective, as lower doses may lead to fewer side effects without compromising efficacy.
Two additional studies of the program were conducted at Stanford and the NCI:
- Phase 1 Trial in Pediatric and Young Adult Patients with R/R B-ALL at Stanford:
- The trial enrolled a younger demographic, including pediatric and young adult patients with R/R B-ALL or LBCL, broadening the scope of CRG-022's evaluation.
- A CR rate of 25% (4 out of 16 treated patients) at Day 28, with different durations of response between adult and pediatric populations, indicates some level of efficacy but also suggests variability in response across age groups.
- A 50% overall survival at one year highlights the potential for CRG-022 to deliver meaningful clinical benefits.
- However, the occurrence of severe CRS, HLH-like syndrome, and a death due to multiorgan failure point to significant safety concerns that need to be carefully managed in this patient group.
- Phase 1 Trial at the NCI
- The trial at the NCI included a larger cohort of patients (73 enrolled) with a high CR rate of 70%, suggesting robust antitumor activity of the CD22 CAR T-cell therapy.
- The high percentage of patients achieving minimal residual disease (MRD) negative status (88% of responders) is particularly promising, as MRD-negativity is often associated with better long-term outcomes.
- The incidence of cytokine release syndrome (CRS) was high (82%), but mostly of lower grade, aligning with the known safety profile of CAR T-cell therapies.
- Neurotoxicity and HLH-like manifestations were observed, with HLH-like manifestations prompting the use of anakinra, an IL-1 receptor antagonist, indicating a proactive approach to managing known CAR T-cell therapy side effects.
Phase 2 study
CARGO recently started dosing in its potentially pivotal Phase 2 study. The Phase 2 study of CRG-022 is a multi-center, open-label trial evaluating its safety and efficacy in LBCL patients refractory to or relapsed from CD19 CAR T-cell therapy. Key design elements include:
- Enrollment: Up to 123 patients with separate cohorts for different prior treatments.
- Primary Endpoint: Objective Response Rate (ORR) as determined by an independent review committee.
- Secondary Endpoints: Efficacy-related measures (like complete response rate, duration of response), safety (incidence rate of adverse events), progression-free survival, and overall survival.
- Treatment Regimen: Lymphodepletion followed by CRG-022 infusion across different cohorts with dosages of 1 x 10^6 cells/kg or 0.1 x 10^6 cells/kg.
- Follow-Up: Efficacy and safety follow-ups at 3, 9, and 24 months post-infusion.
The inclusion and exclusion criteria for the CRG-022 Phase 2 study are designed to ensure a well-defined patient population that can safely participate and likely benefit from the therapy. Key inclusion criteria focus on adults with confirmed LBCL who have previously received CD19 CAR T-cell therapy or two prior lines of therapy including a bispecific T-cell engaging antibody. These criteria are set to select patients who have not responded adequately to existing treatments, positioning CRG-022 as a potential option for those with limited alternatives.
The exclusion criteria are comprehensive, covering various health conditions and treatments that could interfere with the study's safety or the interpretation of efficacy results. Excluding patients with other malignancies, significant comorbidities, CNS involvement, or a history of certain infections helps to minimize risks and potential confounders.
The stringent selection process, alongside defined endpoints such as ORR, duration of response, and overall survival, aims to ensure the reliability and reproducibility of the study outcomes. However, the complexity of the criteria may limit the speed of patient recruitment and could potentially exclude individuals who might benefit from the therapy.
Regulatory agencies will scrutinize the trial design, patient selection, and consistency in manufacturing and administration of the therapy to ensure that the study results are reliable and applicable to a broader patient population. Compliance with these criteria is crucial for the success of the trial and the eventual approval of CRG-022.
Manufacturing
CARGO Therapeutics has made modifications to the manufacturing process of CRG-022, the CD22-targeted CAR T-cell therapy, which was originally produced at Stanford for Phase 1 clinical trials. These changes are aimed at improving manufacturing yields and efficiency for commercial-scale production.
Transitioning from a process suitable for phase 1 clinical trials to one that can support larger phase 2 trials and commercial production is complex. It involves ensuring that the process is scalable while maintaining the quality and characteristics of the CAR T-cells.
Any changes in the manufacturing process need to maintain the biological properties of the CAR T-cells, including their efficacy and safety profile. New processes must have robust quality control measures and require validation to ensure they meet regulatory standards.
Alterations in the manufacturing process could affect the CAR T-cell product's efficacy and safety, potentially impacting clinical outcomes. Changes in manufacturing could lead to variations in cell viability and potency, which are critical for the therapeutic effect of CAR T-cell products.
The FDA must be convinced that the CAR T-cells produced by the new process are comparable to those produced by the Stanford process. Any significant differences could impact regulatory approval and labeling. If the FDA does not accept the comparability data, CARGO might face limitations on using data from the Stanford trials to support their regulatory submissions. Disagreement with the FDA on the comparability of the manufacturing processes could lead to delays in trial progression, approval, and ultimately in product launch.
CARGO has conducted comprehensive analyses to demonstrate that CRG-022 produced with the new commercial process is comparable to that produced for the Stanford Phase 1 trials. Providing a thorough package in their IND application to establish comparability, and being transparent with the FDA about the changes made, will be key to addressing potential regulatory concerns.
Market opportunity
Large B-cell lymphoma (LBCL) is a type of non-Hodgkin lymphoma (NHL), which is the most common hematologic malignancy in adults. LBCL represents an aggressive form of NHL and includes various subtypes such as diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphomas, primary mediastinal B-cell lymphoma (PMBCL), and grade 3B or transformed follicular lymphoma (FL).
LBCL typically presents with rapidly growing tumors in lymph nodes, the spleen, liver, bone marrow, or other organs. Symptoms often include swelling of lymph nodes, fever, night sweats, unexplained weight loss, fatigue, and sometimes organ dysfunction depending on the tumor's location.
The prognosis for LBCL varies based on a variety of factors, including the specific subtype, stage at diagnosis, patient age, and response to initial therapy. Generally, aggressive forms of LBCL require prompt treatment, and the outcome can be poor if the disease is refractory or relapses after standard therapies.
In the United States, NHL accounts for an estimated 80,550 new cases and 20,180 deaths in 2023. Among NHLs, LBCLs account for approximately 30% to 40% of cases.
First-line treatment for LBCL includes a combination of CD20-targeted monoclonal antibodies (like rituximab) and anthracycline-based chemotherapy regimens. Patients who do not respond to initial treatments or relapse may receive salvage chemotherapy followed by high-dose therapy and autologous stem cell transplant (ASCT), provided they are eligible. Recently, CD19-directed CAR T-cell therapies have been approved for use in certain LBCL patients who are refractory to or relapse after other treatments.
Despite the advances in treatment, there remains a significant unmet need for effective therapies in LBCL. This is particularly true for patients who relapse after or are refractory to CD19 CAR T-cell therapies. CD19 antigen loss or downregulation is observed in a substantial proportion of these cases, rendering CD19 CAR T-cell therapies ineffective. Moreover, the prognosis for LBCL patients post CD19 CAR T-cell therapy failure is poor, with median overall survival ranging from approximately five to eight months.
Estimates for relapse or refractoriness to CD19 CAR-T cell therapies can vary based on the specific population being treated, the particular CAR-T product used, and the length of follow-up. However, clinical trials and real-world studies provide some general insights.
In the ZUMA-1 clinical trial for Yescarta, approximately 60% of LBCL patients treated had their disease relapse or progress within 24 months. At the five-year mark, the percentage of patients experiencing disease progression or death increased slightly. Translational studies have suggested that antigen loss or downregulation, which is a key mechanism of resistance, occurs in about 30% to 60% of cases.
Post-marketing data and patient registries indicate that the median overall survival for patients with aggressive B-NHL post CD19-directed CAR T failure is approximately five to eight months, suggesting a significant rate of relapse or refractoriness.
Revenue build: CD19 R/R LBCL
- Target Population: Assume 10,000 LBCL patients are treated with CD19 CAR-T annually, with a 50% relapse/refractory rate, creating a target population of 5,000 patients for CRG-022.
- Market Penetration: Aim for a 10% market penetration in Year 1, increasing by 10% annually to a maximum of 50%.
- Pricing Strategy: Set a placeholder price of $375,000 per treatment, aligning with current CAR-T therapy costs. Apply gross-to-net discounts of 20%.
- Insurance Coverage: Assume 90% coverage with copay assistance programs covering most patient out-of-pocket costs.
- Duration of Therapy: Account for a one-time infusion with a placeholder assumption of 2-year average remission duration.
Revenue build: CAR-T naive LBCL
- Target Population: Assume 10,000 LBCL patients are treated with CD19 CAR-T annually.
- Market Penetration: Aim for a 10% market penetration in Year 1, increasing by 10% annually to a maximum of 50%.
- Pricing Strategy: Set a placeholder price of $375,000 per treatment, aligning with current CAR-T therapy costs. Apply gross-to-net discounts of 20%.
- Insurance Coverage: Assume 90% coverage with copay assistance programs covering most patient out-of-pocket costs.
- Duration of Therapy: Account for a one-time infusion with a placeholder assumption of 2-year average remission duration.
Competition
CRG-022 will face substantial competition from both existing approved therapies and those in development. The competitive landscape for CAR-T therapies in LBCL includes:
- Approved CD19 CAR T-cell Therapies
- Axicabtagene Ciloleucel (Yescarta): With strong clinical trial results showing an ORR of 83% and a CR rate of 54% after two years, and 32% progression-free survival (PFS) after five years, Yescarta sets a high efficacy benchmark for CRG-022.
- Tisagenlecleucel (Kymriah): Demonstrating an ORR of 52% and a CR rate of 38% after two years, Kymriah also presents significant competition, especially considering its PFS of 31% after 36 months.
- Lisocabtagene Maraleucel (Breyanzi): With an ORR of 73% and a CR rate of 53% after two years, Breyanzi shows comparable efficacy to Yescarta and provides another strong market competitor.
- Allogeneic CAR T-cell Therapies in Development
- Companies like Allogene Therapeutics and Atara Biotherapeutics are developing "off-the-shelf" allogeneic CAR T-cell therapies that could offer logistical advantages over autologous therapies like CRG-022, such as shorter production times and potentially lower costs.
- Autologous CAR T-cell Therapies in Development
- Other companies are developing autologous therapies that target different antigens or the same antigens with different constructs, which could provide alternative options for patients who are refractory or relapsed from CD19 CAR-T therapies.
There are over 100 CAR-T blood cancer programs in clinical trials, including dozens of next-generation approaches. Some approaches are described below:
IP and license agreements
IP
CARGO Therapeutics' intellectual property (IP) strategy is integral to its business, relying on patents, trademarks, trade secrets, and contractual agreements to safeguard its innovations. As of August 2023, they have six pending U.S. and two PCT applications covering various technologies related to cell therapies, with patents potentially expiring between 2043-2044. Exclusively licensed from NCI and Stanford, they also have a portfolio of six U.S. patents, four pending U.S. applications, international patents, and applications expected to expire from 2029 to 2042.
CARGO's portfolio includes innovations in cytokine receptor switches, chimeric antigen receptors (CARs), and methods for enhancing immunotherapies. The company's commercial success hinges on securing effective patent claims and protecting against infringement, while navigating the uncertainties of patent issuance and enforcement. Their proactive approach to IP management includes engaging in potential litigation to enforce or defend their IP rights, which are vital for maintaining a competitive edge in the market. Despite robust IP controls, the company is aware that litigation can be costly and the compensation from such actions may not fully offset the damages caused by infringement.
License agreements
Stanford license agreement
CARGO Therapeutics entered into an exclusive licensing agreement with Stanford University in August 2022, providing access to certain patents and technology for human therapeutic and diagnostic products. They paid Stanford $50,000 upfront, issued 67,605 common stock shares, and will pay up to $100,000 in annual fees, $7.5 million in sales milestones, $3.98 million in development milestones, and $550,000 upon achieving commercial milestones. They also agreed to low single-digit royalties on net sales and a percentage of sublicensing revenues. The agreement can be terminated by Stanford for material breach or by CARGO with 30 days’ notice, and includes a $250,000 payment to Stanford if CARGO is acquired or sells the relevant assets.
Oxford license and supply agreement
Under the June 2022 license and supply agreement with Oxford Biomedica, CARGO Therapeutics obtained a non-exclusive, worldwide license to use certain lentiviral vectors for developing and commercializing CD22-targeting CAR T-cell therapies, with options for additional targets. They paid Oxford Biomedica an upfront fee of $200,000, with potential milestone payments totaling up to $13.55 million plus royalties on net sales. CARGO retains rights to any new intellectual property developed from using Oxford Biomedica's materials. The agreement remains in effect until all payments are complete and can be terminated with a 120-day notice or due to breach or insolvency.
2022 National Cancer Institute license agreement
The 2022 NCI License Agreement grants CARGO Therapeutics an exclusive worldwide license to commercialize autologous CD22 CAR T immunotherapies for B-cell malignancies, with a separate exclusive option for allogenic therapies. CARGO paid the NCI a $550,000 license fee, with additional potential regulatory, development, and sales milestone payments totaling up to $17.95 million, plus royalties on net sales. They also agreed to share a percentage of sublicensing revenues and PRV sales. The license expires with the last patent right or can be terminated earlier by either party under certain conditions, including breach or insolvency.
2023 National Cancer Institute license agreement
The 2023 NCI License Agreement provides CARGO Therapeutics an exclusive license for certain CAR T immunotherapies targeting B-cell malignancies. They are to pay the NCI a total of $250,000 in fees, up to $1.815 million in regulatory and development milestones, and up to $16 million in sales milestones, along with royalties on net sales. CARGO also agreed to share a percentage of sublicensing revenues and payments related to PRVs. The license remains valid until the last patent expires and can be terminated by either party under certain conditions, including breach or insolvency.
Analyze your company with our AI tools
Get an analysis like this one for your company or portfolio. Includes qualitative analysis and excel files with valuation analysis.
Contact us to learn more.