Carmot Therapeutics investment analysis
December 4, 2023
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Overview
Carmot Therapeutics is a clinical-stage biotechnology company developing innovative therapeutics for metabolic diseases such as obesity and diabetes, conditions that affect over 750 million people worldwide. Recognizing the limitations of current treatments, including issues with tolerance, dosing, and administration, Carmot aims to address these through its pipeline of novel incretin agonists.
The company's research is founded on the Chemotype Evolution platform, which has generated a portfolio of entirely owned drug candidates targeting metabolic hormones GLP-1 and GIP, crucial for energy homeostasis.
Carmot Therapeutics' clinical-stage pipeline includes three product candidates:
-
CT-388: A once-weekly subcutaneous injectable dual GLP-1/GIP receptor agonist for obesity and type 2 diabetes (T2D), demonstrating significant weight loss and favorable tolerance in early trials. Phase 1/2 data on dosing and titration are expected by the first half of 2024, with more results anticipated in 2025.
-
CT-996: A once-daily oral small molecule GLP-1 receptor agonist designed for daily dosing and tolerance in line with the class standards of GLP-1 receptor agonists, specifically regarding gastrointestinal-related adverse events. Initial Phase 1 data are expected in the first half of 2024, with further Phase 1b data on T2D in late 2024.
-
CT-868: A once-daily subcutaneous injectable dual GLP-1/GIP receptor agonist for treating type 1 diabetes (T1D) in patients with overweight or obesity. The candidate has shown significant HbA1c improvements in a 26-week Phase 2 trial for T2D. Ongoing Phase 1 trials are assessing glucose homeostasis in T1D, with initial data expected in early 2024 and a Phase 2 proof-of-concept trial underway, projecting data for the second half of 2024.
Carmot Therapeutics is also advancing preclinical programs targeting PYY, a peptide for potentially treating Prader Willi syndrome and other metabolic diseases in conjunction with other incretin agonists.
Carmot's product differentiation lies in its tailored action on incretin pathways, fundamental to metabolic control. The integration of dual agonists could provide comprehensive metabolic regulation, potentially leading to superior outcomes like enhanced weight loss and glycemic control. Furthermore, development of oral agents like CT-996 addresses a significant market demand for non-injectable therapies, potentially improving patient compliance.
Pipeline overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| CT-388 | Peptide | GLP-1/GIP Agonist | Obesity | |||||||
| CT-388 | Peptide | GLP-1/GIP Agonist | Type 2 Diabetes | |||||||
| CT-868 | Peptide | GLP-1/GIP Agonist | Type 1 Diabetes with overweight or obesity | |||||||
| CT-996 | Small molecule | GLP-1 Agonist | Obesity | |||||||
| CT-996 | Small molecule | GLP-1 Agonist | Type 2 Diabetes | |||||||
| CT-PYY | Peptide | PYY Analog | Prader Willi syndrome |
Highlights and risks
GLP-1 agonists have the potential to become some of the best selling drugs of all time
Targeting very large and rapidly growing markets
Potential differentiation in dual-acting mechanism, biased signaling, and oral delivery
GLP-1/GIP dual agonists have demonstrated greater weight loss than GLP-1 mono-agonists
Positive early clinical data
Weight loss observed in early clinical trials of CT-388 appears to be lower than that of approved GLP-1 and GLP-1/GIP agonists
Will need to demonstrate some level of clinical differentiation, or comparable clinical activity and lower cost, to garner significant revenue
Late-stage trials in Type 2 Diabetes and obesity are very expensive
Drugs will need to generate billions in annual sales to justify price paid
Valuation
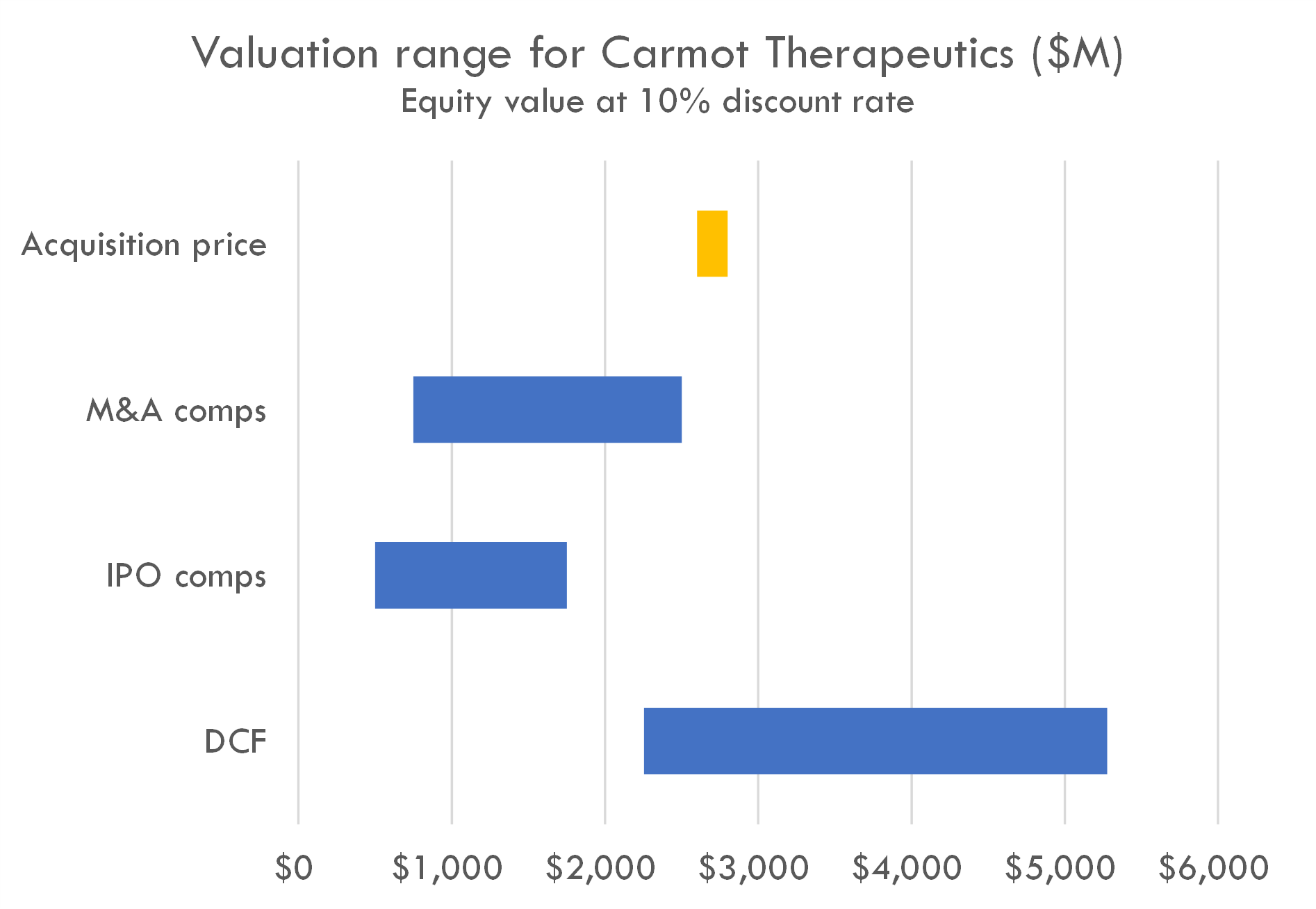
Carmot Therapeutics was acquired by Roche for $2.7 billion in December 2023. Before the acquisition, Carmot had filed to go public.
Our valuation is based on IPO comps M&A comps, and a DCF analysis.
Our DCF uses a 10% discount rate representing the acquiror's discount rate. Our model uses the probability of success and revenue build assumptions outlined below. Our peak sales for CT-388 ranges from $3 billion to $16 billion, with base case peak sales of $10 billion. Our peak sales estimate for CT-868 is $720 million. We estimate peak annual sales for CT-996 of $10 billion.
GLP-1 agonists overview
GLP-1 agonists have significantly impacted the treatment of type 2 diabetes and obesity. Clinical trials reveal that semaglutide (Wegovy) achieves around 12% weight loss, while tirzepatide (Mounjaro) leads to approximately 18%. Semaglutide also notably decreases cardiovascular events by 20% and improves heart failure symptoms. Their commercial potential is robust, with sales for Ozempic/Wegovy and Mounjaro projected to reach $31.3 billion and $28.5 billion by 2030. Despite challenges like tolerability and complex dosing, these drugs are crucial in managing metabolic diseases.
CT-388
Scientific thesis
The therapeutic rationale for a GLP-1/GIP receptor agonist in obesity and type 2 diabetes (T2D) is rooted in the understanding of how these hormones regulate glucose metabolism and energy balance.
GLP-1 (Glucagon-Like Peptide-1):
- Glucose-Dependent Insulin Secretion: GLP-1 enhances the secretion of insulin from pancreatic beta cells in a glucose-dependent manner. This means it promotes insulin secretion only when blood glucose levels are already elevated, reducing the risk of hypoglycemia (dangerously low blood sugar levels).
- Suppression of Glucagon: It also suppresses glucagon secretion, which otherwise would raise blood glucose levels.
- Satiety and Reduced Caloric Intake: GLP-1 contributes to satiety, leading to reduced food intake and potentially aiding in weight loss.
- Gastric Emptying: It slows gastric emptying, contributing further to satiety and helping to control postprandial (after-meal) glucose excursions.
GIP (Glucose-Dependent Insulinotropic Peptide):
- Insulin Secretion: Like GLP-1, GIP also stimulates insulin secretion in a glucose-dependent manner.
- Lipid Metabolism: It plays a role in lipid metabolism and potentially in energy expenditure and fat distribution.
By combining GLP-1 and GIP receptor agonistic activities in one therapeutic (like CT-388), there could be several beneficial outcomes:
- Enhanced Glycemic Control: The dual mechanism can lead to improved blood glucose regulation by increasing insulin secretion and reducing glucagon secretion more effectively than either agonist alone.
- Incremental Weight Loss: GLP-1 reduces food intake, and both peptides could have additive effects on weight reduction due to their combined influence on satiety and possibly energy expenditure.
- Potentially Improved Tolerability: By tweaking the drug to favor certain signaling pathways that are less associated with side effects, there may be improved tolerability, leading to better adherence to therapy.
For CT-388 specifically, the product candidate has been designed to signal through cAMP pathway with minimal to no recruitment of ß-arrestin. Biased signaling has been hypothesized to confer a therapeutic advantage because ß-arrestin recruitment can lead to receptor internalization and desensitization, which may dampen a drug's therapeutic effects over time. By minimizing ß-arrestin involvement, CT-388 might maintain potent pharmacological activity for an extended duration, which is favorable for a chronic condition such as T2D, where long-term treatment is required.
Moreover, the once-weekly formulation adds to patient convenience, potentially increasing adherence to the medication regimen. With the observed significantly greater weight loss and glycemic control compared to mono GLP-1 receptor agonists—as seen in clinical trials comparing drugs like tirzepatide and semaglutide—the dual agonists are positioned as promising next-generation therapeutics for obesity and T2D.
Clinical trial data for CT-388 has so far indicated substantial weight loss and favorable tolerability, aligning with the expected therapeutic benefits of this approach. The strategic development of CT-388 is reflective of an iterative, knowledge-driven process that combines detailed understanding of GPCR signaling with the practical demands of effective metabolic disease treatment.
In summary, the therapeutic rationale for dual GLP-1/GIP receptor agonists in the treatment of obesity and Type 2 Diabetes is to harness and enhance the physiological roles of these incretins in glucose regulation and weight management, while optimizing the drug's signaling to improve efficacy, duration of action, and tolerability.The science underpinning the use of GLP-1 and GIP receptor agonists in the treatment of obesity and type 2 diabetes (T2D) is well-established, but some aspects are still subject to ongoing research and debate. Here’s a summary of the current level of evidence and areas of uncertainty:
Well-Established Evidence:
GLP-1 and Glycemic Control: GLP-1’s role in enhancing insulin secretion, suppressing glucagon release, and slowing gastric emptying is well-documented in studies and clinical practice.
GLP-1 and Weight Loss: The effects on satiety and caloric intake contribute to GLP-1 agonists' efficacy in weight reduction, supported by numerous clinical trials.
GIP’s Role in Insulin Secretion: GIP also promotes insulin secretion in response to oral glucose load, which is a known physiological role.
Benefits of GLP-1 Agonists in T2D and Obesity: The clinical benefits of GLP-1 agonists like liraglutide and semaglutide for glucose control and weight loss in T2D and obesity are supported by extensive clinical data, including results from randomized controlled trials.
Areas of Ongoing Research and Debate:
GIP’s Effects on Weight: While GLP-1's effects on weight are well-recognized, the exact role of GIP in body weight regulation is less clear. Some studies suggest that GIP may promote energy storage, while others indicate potential weight loss benefits, particularly when combined with GLP-1 agonism.
Biased Agonism: The concept of biased agonism - where a drug preferentially activates one signaling pathway over another - is a relatively new area in pharmacology. While preclinical studies support that biased agonism may lead to prolonged pharmacologic responses and decreased side effects, more research is needed in this area, especially to fully understand the clinical implications.
Comparative Efficacy of Dual Agonists: Comparisons between mono and dual agonists, such as tirzepatide (dual GLP-1/GIP agonist) versus semaglutide (GLP-1 agonist), indicate that dual agonists may be superior in terms of weight loss and glycemic control. However, understanding the independent contributions of GLP-1 and GIP receptor activation to the overall therapeutic effect still requires more investigation.
ß-Arrestin’s Role: The role of ß-arrestin in the desensitization of GLP-1 and GIP receptors and its impact on the long-term efficacy and safety of receptor agonists are still being explored.
Overall Level of Evidence:
The clinical use of GLP-1 receptor agonists in the management of T2D and obesity is based on a high level of evidence, while the therapeutic rationale for GIP receptor agonism is on firmer ground but not as extensively validated as that for GLP-1.
Biased agonism, as a strategy for improving drug efficacy and tolerability, represents a more cutting-edge aspect of endocrine pharmacotherapy and, while promising, is still under investigation. Clinical trial results, such as those discussed for investigational drugs like CT-388, will further contribute to the scientific understanding and validation of biased agonism in the treatment of metabolic diseases.
The literature on GLP-1 (Glucagon-Like Peptide-1) and GIP (Glucose-Dependent Insulinotropic Polypeptide) in the context of obesity and Type 2 Diabetes (T2D) includes numerous studies that highlight their significant roles and therapeutic potential. Below are some key examples from the scientific literature:
GLP-1 and Obesity/T2D:
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117(1):24-32. This review discusses the biology of GLP-1 and its role in controlling blood glucose levels and body weight, which has been foundational in the development of GLP-1-based therapies for T2D and obesity.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46-52. This early study describes the impaired incretin effect in T2D patients, laying the groundwork for understanding the therapeutic potential of incretin hormones.
- Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317-32. This review compares the efficacy and safety of GLP-1 receptor agonists for the treatment of T2D, confirming their significant role in current therapeutic strategies.
GIP and Obesity/T2D:
- Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5-21. This article summarizes the role of incretin hormones, including GIP, in metabolic regulation and the treatment of T2D.
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515-20. Although this study focuses on GLP-1, it's relevant in supporting the incretins' role in satiety and food intake regulation.
Dual GLP-1/GIP Agonism:
- Friis L, Glendorf T, Søndergaard FL, et al. A long-acting dual GIP/GLP-1 receptor agonist with improved activity against GIP resistance. Biochem J. 2020;477(21):4107-4124. This study explores a long-acting dual GLP-1/GIP receptor agonist, discussing its enhanced activity against GIP resistance, which often occurs in T2D.
- Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3-14. This paper presents discovery and development of a dual receptor agonist (later known as tirzepatide), discussing preclinical and early clinical evidence of its utility in T2D treatment.
Clinical Trials:
- Pratley R, Aroda V, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275-286. This study provides clinical trial data for GLP-1 receptor agonists in T2D.
- Surmontil-1 Clinical Trials: While specific published studies for the SURMOUNT-1 trial might not be available until after my knowledge cut-off, Trial Identifier: NCT03548935 would be the starting point for data on the clinical trial results of tirzepatide, which might provide evidence supporting the dual agonist approach.
These literature sources provide a solid background for the roles of GLP-1 and GIP in the context of obesity and T2D and the clinical rationale for their use as therapeutic targets in these conditions. Additionally, the evidence from ongoing clinical trials continues to accumulate, further informing the therapeutic potential and optimization of incretin-based therapies.
The evidence base supporting the therapeutic rationale for GLP-1 and GIP receptor agonists in obesity and Type 2 Diabetes (T2D) involves a mix of basic research, translational studies, clinical trials, and real-world evidence. Here is an analysis of the strengths and weaknesses:
Strengths:
Robust Clinical Data for GLP-1 Receptor Agonists: The efficacy and safety of GLP-1 receptor agonists are supported by high-quality evidence from numerous randomized controlled trials (RCTs) and meta-analyses. Such drugs are now standard care in modern T2D treatment guidelines.
Mechanistic Understanding: There is a strong biological rationale based on an understanding of the physiological roles of GLP-1 and GIP on insulin secretion, glucose regulation, and satiety.
Diverse Patient Populations: Clinical trials of GLP-1 receptor agonists have been conducted in a wide range of patient populations, including those with varying severities of obesity and T2D, enhancing the generalizability of findings.
Long-term Studies: Several long-term studies provide evidence for the durability and sustained effects of GLP-1 receptor agonists on glycemic control and body weight.
Positive Outcomes Beyond Glycemic Control: GLP-1 receptor agonists have been shown to have cardiovascular benefits, a significant consideration since T2D is a risk factor for heart disease.
Innovative Clinical Development: The clinical development of dual GLP-1/GIP receptor agonists like tirzepatide has provided promising results, potentially indicating superior efficacy over GLP-1 receptor agonists alone in early-phase and pivotal clinical trials.
Weaknesses:
Emerging Evidence for GIP Receptor Agonists: Evidence for therapeutic use of GIP is less established compared to GLP-1. Dual agonists are newer, and the added benefit of GIP on top of GLP-1 is less well-understood.
Concerns Over Biased Agonism: The concept of biased agonism as a beneficial mechanism in incretin therapies is still not entirely proven. While preclinical data are promising, clinical evidence directly attributing benefits to reduced β-arrestin recruitment is still accruing.
Complexity of Endocrine Physiology: The enteroendocrine system is complex and incompletely understood. This may lead to unforeseen effects as incretin therapies affect an extensive network of pathways.
Adverse Effects and Tolerability: Despite promising tolerability profiles, incretin therapies, particularly GLP-1 receptor agonists, can cause gastrointestinal side effects, which may limit their use in some patients.
Long-term Safety Data: The long-term safety profile, especially for newer agents like dual GLP-1/GIP receptor agonists, has yet to be fully established as they have been studied for a relatively short period.
Cost and Accessibility: Incretin therapies, especially those with novel mechanisms like dual agonists, are often more expensive than other antidiabetic medications, which can affect accessibility for many patients.
Overall, the evidence base is strong for GLP-1 receptor agonists in the management of obesity and T2D, with emerging evidence supporting the potential additional benefits of dual GLP-1/GIP receptor agonists. However, as with any therapeutic domain that involves novel mechanisms of action and new pharmacological entities, ongoing research and further long-term studies are necessary to fully characterize efficacy, safety, and best-use scenarios for targeted patient groups.
Clinical trial overview
The study for CT-388 is a Phase 1 clinical trial that aims to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of a drug intended for the treatment of obesity and Type 2 Diabetes Mellitus (T2DM). This is a first-in-human study involving CT-388.
Summary of study design:
- The trial is designed to be double-blind, meaning neither the participants nor the investigators nor the outcomes assessors know who is receiving the active treatment or the placebo.
- It is placebo-controlled, which means the control group receives a placebo to compare against the effects of the drug.
- It is randomized to ensure participants are assigned to intervention or placebo groups by chance, reducing bias.
- The study uses a sequential assignment, which may imply a stepwise enrollment where different cohorts might receive different dosages or conditions at different times.
- It involves healthy obese adults and obese adults with T2DM, indicating a focus on both the general obese population and a subset with a specific metabolic disorder.
Schedule and size:
- Started in April 2021, with primary completion estimated for September 2023 and study completion the following month.
- The estimated enrollment is 96 participants.
Outcomes:
- Primary outcome is the incidence of treatment-emergent adverse events, assessing safety and tolerability from baseline up to 6 weeks.
- Secondary outcomes include PK measures such as AUC, Cmax, and half-life, and PD measures such as changes in body weight, glucose levels, and insulin levels.
Critiques of the study design:
- The sequential assignment is not thoroughly detailed in the summary and could introduce bias or affect the trials' blinding if not properly managed.
- While the enrollment size seems reasonable for a Phase 1 trial, it may not fully represent the diverse populations affected by obesity and T2DM.
- A 6-week duration may be limited to assess longer-term safety, tolerability, and metabolic effects of CT-388, particularly for a condition like diabetes that requires long-term management.
- It is also not clear whether the trial will evaluate the potential impact of the drug on comorbid conditions often associated with obesity and T2DM, such as cardiovascular disease.
Operational and technical challenges:
- Ensuring strict adherence to the double-blind procedure can be operationally challenging, especially if the sequential assignment leads to different interventions over time, which should be managed carefully to maintain the integrity of blinding.
- The assessment of safety and tolerability relies on self-reporting, which can introduce subjective bias; therefore, it's important to complement it with objective measures and validate the reporting methods.
- Subcutaneous injections require training and skill to administer, which might affect the consistency of the intervention across the study population.
- Maintaining participant retention over the course of the study could be challenging, particularly if adverse events are significant.
Overall, while the design of the phase 1 study for CT-388 is within standard protocols for new therapeutic agents, certain details of the design and operational aspects would benefit from further clarification. The study appears to be well-structured to assess preliminary safety and efficacy, setting the foundation for more expansive Phase 2 and Phase 3 trials.
Primary and secondary endpoints: For a POC study, the chosen primary outcome measures, specifically the incidence of treatment-emergent adverse events, are appropriate as ensuring the drug is safe at this stage is critical. The secondary outcomes related to PK (AUC, Cmax, and half-life) and PD (changes in body weight, glucose levels, and insulin levels) measures are also fitting, as they will help establish the dose-response relationship, exposure levels, and preliminary efficacy of CT-388. These measures are standard and relevant for characterizing both the metabolic effects (which would indicate efficacy in reducing obesity and managing T2DM) and the pharmacologic profile of the drug. They are critical for determining if CT-388 has potential therapeutic benefits.
Inclusion/exclusion criteria: The criteria for inclusion are quite standard for clinical trials in this field:
- Age: Participants between 18 and 65 include most adults at risk for obesity and T2DM but exclude older adults who may have different pharmacodynamics and comorbidities.
- BMI Range: The chosen range of 27.0–40.0 includes individuals who are overweight or obese (according to WHO classifications) but not severely or morbidly obese, which might introduce additional medical complications.
- Stable body weight: This helps ensure that any change can more likely be attributed to the drug instead of other factors.
- Sex: The study accepts all sexes, which is important for generalizability.
The exclusion criteria:
- No significant medical history, uncontrolled hypertension, or history of malignancy: These conditions could complicate the interpretation of the drug's effects or pose additional risks to the participants.
The criteria chosen are appropriate to minimize confounding variables and safety risks while providing a clear POC for efficacy and safety in a typical population of patients with obesity and at higher risk of T2DM. However, the exclusion of those with 'significant medical history' is a broad term that requires clarification. If this term is not clearly defined in practical, operational terms (e.g., specific diseases or conditions that constitute a 'significant medical history'), there could be variability in how it's applied, which raises concerns about reproducibility and the generalizability of the results.
Reproducibility challenges: Strict inclusion and broad exclusion criteria may limit enrollment and the representativeness of the study population to the general disease population. For example, excluding those with uncontrolled hypertension might limit understanding of how the drug affects a significant subset of the obese population, which often experiences hypertension. Also, individuals with significant medical histories may be the most in need of efficacious treatments for obesity and T2DM but are excluded here, which could impact the reproducibility of these results in later trials where such participants might not be excluded.
If the study proves that CT-388 is safe and shows a potential beneficial effect on body weight, glucose, and insulin levels within the controlled environment of this trial, it could lead to a Phase 2 trial. However, the demographic might be expanded in Phase 2 to include a broader patient population and assess the drug's efficacy and safety across a wider spectrum of the patient population typically affected by obesity and T2DM.
Clinical trial results
The clinical data supporting CT-388 for the indication of obesity in a Phase 2 context can be summarized from the completed parts of a multi-arm, Phase 1/2 clinical trial. Below is a summary of the relevant clinical findings:
Safety and Tolerability CT-388 was generally well tolerated in both single ascending dose (SAD) and multiple ascending dose (MAD) portions of the trial. The most common treatment-emergent adverse events (TEAEs) were gastrointestinal (GI) in nature, which aligns with the known side effects of incretin-based therapies. These TEAEs were mostly mild to moderate (Grade 1 or 2). There were no discontinuations due to TEAEs, indicating an acceptable safety profile at the doses tested.
Pharmacokinetics (PK) The PK profile demonstrated a dose-proportional increase in maximum concentration (Cmax) and area under the curve (AUC) supporting once-weekly dosing with a mean half-life of approximately 150 hours.
Efficacy Significant and dose-proportional weight loss was observed across the cohorts receiving CT-388 in the MAD portion of the trial, with up to an average of 8.4% weight loss noted in the highest dosed cohort (8) after four weeks. The weight loss was rapid, observed as early as Day 8, and persisted through the study duration. Notable reductions in waist circumference were observed across all cohorts, indicative of a reduction in visceral body fat, which is associated with better metabolic health and decreased insulin resistance. Insulin sensitivity, assessed by the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), improved with the administration of CT-388, particularly in the highest dosed cohort.
Additional Observations Demographic data (e.g., average age, gender distribution, and ethnicity) were provided for cohorts 6, 7, and 8. A decrease in appetite was noted as a metabolism and nutrition disorder TEAE in 100% of CT-388 treated participants but not reported among those who received a placebo. Tolerability was suggested to be more favorable in participants with obesity compared to those with overweight.
Ongoing and Future Development The study is continuing with further cohorts to refine the dosing and titration scheme for upcoming Phase 2 trials. Future trials will explore different dosing and duration (up to 36 weeks) and are intended to provide data to support CT-388's development for obesity and Type 2 diabetes (T2D).
Potential for Broader Indications Given that obesity is linked with numerous comorbidities, CT-388 could have a range of potential future indications beyond obesity and T2D, such as nonalcoholic steatohepatitis (NASH), cardiovascular diseases (CVD), heart failure, osteoarthritis, sleep apnea, and chronic kidney disease (CKD).
Overall, CT-388 has yielded promising results in terms of safety, tolerability, and efficacy for the treatment of obesity, with data supporting further clinical development and exploration at higher doses. The ongoing studies are expected to provide more detailed information for optimizing dosing regimens and expanding the therapeutic potential of CT-388.
Clinical Data from Competitor Agents:
- Incretins, specifically GLP-1 and GIP, are well-established in treating diabetes and obesity. They stimulate insulin secretion, inhibit glucagon secretion, and promote satiety, among other effects.
- Exenatide (Byetta) was the first incretin therapy approved for T2D, and liraglutide (Saxenda) was the first long-acting GLP-1 receptor agonist approved for weight management.
- Several long-acting GLP-1 receptor agonists, including semaglutide (Wegovy) and the dual GLP-1/GIP receptor agonist tirzepatide (Mounjaro), are FDA-approved or under review for obesity treatment.
- Semaglutide has demonstrated significant weight loss (approximately 12% net) and cardiovascular benefits in clinical trials. Wegovy (semaglutide) showed a 20% reduction in major adverse cardiovascular events over five years.
- Tirzepatide showed even greater efficacy with a placebo-adjusted net weight loss of approximately 18% over 72 weeks in the SURMOUNT-1 trial.
- Data indicate that patients struggle to achieve weight loss goals with diet and exercise alone, but see substantial improvements with incretin therapies.
- Market estimates for these therapies predict considerable growth, highlighting their commercial success and the validation of their targets.
Strengths and Limitations of CT-388 in Light of Competitor Data:
Strengths:- CT-388 demonstrates a positive safety and tolerability profile with manageable GI-related side effects that are consistent with the incretin class.- CT-388 has shown statistically significant weight loss across cohorts in its Phase 1/2 trial, with the highest dose cohort (Cohort 8) averaging an 8.4% weight loss at four weeks.- The prolonged half-life of CT-388 suggests the potential for once-weekly dosing, which could bolster adherence compared to therapies requiring more frequent administration.- Weight loss with CT-388 is accompanied by improvements in metabolic parameters such as waist circumference and HOMA-IR, indicating a potential benefit for patients with obesity and associated comorbidities.
Limitations:- CT-388 data is early stage (Phase 1/2), making comparisons to late-stage or approved competitors regarding efficacy somewhat premature.- Competitor trials involving semaglutide and tirzepatide are based on larger patient populations and longer duration, providing a more robust data set validating their therapeutic benefit.- Weight loss achieved with GLP-1 receptor agonists such as semaglutide and the dual GLP-1/GIP receptor agonist tirzepatide appear greater than the weight loss observed to date with CT-388.- Competitor agents have established cardiovascular benefits and substantial net weight reductions, which set a high efficacy bar for CT-388 to meet or exceed.- Incretin therapies are challenged by supply shortages and complex titration schemes; CT-388 must demonstrate advantages over these and international market challenges to gain wide acceptance.
In conclusion, while CT-388 has shown promise in its early clinical evaluations, there is still much work to be done to match or exceed the established clinical benefits of competitor incretin therapies. The future development of CT-388 will need to focus on demonstrating strong efficacy, addressing the limitations of current therapies, and proving its value in a competitive market.
For the treatment of obesity, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) usually require specific endpoints to be met in clinical trials for a drug to be considered for approval. CT-388, being in Phase 2 for the treatment of obesity, would need to demonstrate efficacy and safety through well-defined endpoints.
Possible Approvable Endpoints for Obesity:
- Weight Loss Efficacy:
- Absolute weight loss (in kg or lbs) from baseline.
Percentage of weight loss from baseline. The FDA typically requires a drug to produce a mean weight loss that is at least 5% greater than placebo at one year. In addition, the proportion of subjects who lose at least 5% and 10% of their baseline body weight is often evaluated.
Waist Circumference Reduction:
Reduction in waist circumference from baseline, which is an indirect measure of visceral fat and is associated with a lower risk of cardiovascular comorbidities.
Cardiometabolic Health Improvements:
Changes in cardiometabolic risk factors such as blood pressure, lipid profile (e.g., LDL, HDL, triglycerides), glucose control (e.g., fasting glucose, HbA1c), and insulin sensitivity (e.g., HOMA-IR).
Patient-Reported Outcomes:
- Improvement in quality of life measurements using standardized and validated instruments.
Assessment of the impact of weight loss on function and mobility.
Durability of Response:
Sustained weight loss over a longer duration, usually evaluated at 1 year or more.
Safety and Tolerability:
- Frequency, severity, and type of adverse events compared to placebo.
- Discontinuation rates due to adverse events.
- Long-term safety data.
Clinical Studies for Approval:
- Pivotal Phase 3 Trials:
- After achieving positive results in Phase 2, CT-388 would need large-scale Phase 3 trials to confirm its efficacy and safety.
- These trials should be randomized, double-blind, placebo-controlled, and adequately powered to show a statistically significant difference between CT-388 and placebo.
Long-term trials (at least one year) are required for chronic treatments like obesity therapies to demonstrate sustained efficacy and to monitor for adverse effects.
Dose-Response Studies: Further studies may be required to determine the optimal dosing of CT-388 that balances efficacy and safety.
Diverse Population Studies: Trials should include diverse patient populations, especially including individuals at risk for obesity-associated comorbidities, to ensure generalizability and to assess efficacy and safety across different subgroups.
Post-Marketing Surveillance: If approval is granted, post-marketing studies are often required to monitor the drug's performance in a real-world setting, tracking long-term safety and efficacy.
Comparative Effectiveness Trials: While not always required for initial approval, trials that compare CT-388 to other standard obesity treatments can strengthen the drug's positioning in the market.
The endpoints and study design for Phase 3 trials of CT-388 would need to align with regulatory requirements, typically involving discussions with the FDA or EMA. Such discussions help refine the objectives and methodologies that will satisfy agency demands for proving the drug's value over currently available treatments. The successful development of CT-388 would be predicated not just on its ability to induce weight loss, but also to improve overall metabolic health and to be tolerated well by patients over long-term treatment courses.
Market overview
Obesity
The market opportunity in obesity is significant considering the high prevalence and associated health complications, which include type 2 diabetes (T2D), cardiovascular diseases (CVD), sleep apnea, chronic kidney disease (CKD), certain cancers, and an increased risk of death. Worldwide, obesity is one of the most common chronic diseases and affects over 750 million people. The prevalence has tripled since 1975 and is projected to affect one billion people globally by 2030. In the United States, 74% of adults are overweight or obese, with 42% of adults being obese. The economic burden is also considerable, with an estimated total annual cost of chronic diseases caused by obesity and excess weight at $1.72 trillion in the U.S. alone in 2016.
With T2D being a chronic disease with sustained high blood glucose levels and affecting around 400 million people worldwide, and with approximately 90% of T2D cases being associated with overweight or obesity, obesity presents a primary target for preventative healthcare measures and treatments.
The relationship between obesity and T2D is clear, with obesity being a leading risk factor for the development of T2D. Weight loss has been shown to result in improvements in many comorbidities associated with obesity and T2D. Once an individual is obese, it becomes challenging to lower weight with exercise and diet alone, highlighting the need for effective medical interventions.
When looking at the pharmaceutical market, successful drugs for the treatment of obesity have typically demonstrated effectiveness in not just weight loss, but also the improvement of obesity-related conditions, particularly T2D. Some of the successful drugs include:
Orlistat (marketed as Xenical and Alli): Orlistat inhibits pancreatic lipase, reducing fat absorption.
Phentermine/topiramate (Qsymia): This combination drug suppresses appetite and increases satiety.
Naltrexone/bupropion (Contrave): This drug combination acts on the central nervous system to increase satiety and reduce appetite.
Liraglutide (Saxenda): Initially developed for T2D, liraglutide was found to help with weight loss as well.
Semaglutide (Wegovy): Also initially for T2D (as Ozempic), semaglutide was approved for chronic weight management in adults who are obese or overweight.
The standard of care for obesity typically includes lifestyle modifications such as diet and exercise. When these are insufficient, pharmacotherapy may be considered, particularly for individuals with a BMI greater than 30 or those with a BMI greater than 27 who have obesity-related comorbidities. For T2D, first-line treatments include lifestyle interventions and metformin, with insulin and various other medications used as the disease progresses.
The unmet medical need in obesity is substantial, as many patients struggle with weight management despite available treatments. There remains a need for interventions that are more effective, have fewer side effects, are easier to use (e.g., oral versus subcutaneous), and provide sustained weight loss while also minimizing and treating obesity-related conditions. Drugs that can achieve these outcomes have a strong market potential, given the significant direct and indirect costs associated with obesity and T2D.
Furthermore, new treatments that offer a better safety profile, adherence benefits (such as once-weekly dosing or non-invasive administration routes), and that can prevent the progression or development of obesity-related diseases can capitalize on this opportunity. As such, companies investing in the research and development of new obesity treatments are addressing a substantial and growing global health issue with significant market potential.
There are several promising treatments in development for obesity that are positioned to compete with existing therapies if they gain approval:
CT-388 is a product candidate that would likely compete with currently approved injectable products for people with obesity or T2D in the GLP-1 and GLP-1 combination class. Key competitors for CT-388 include Wegovy for obesity, Ozempic for T2D, Trulicity (dulaglutide) for T2D, and Mounjaro for T2D and potentially obesity (currently under review). There are several injectable peptide products pursuing similar indications with similar mechanisms of action from companies such as Altimmune, Viking, Jiangsu Hengrui, and Viking. Additionally, there are combination products in development from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sciwind Biosciences.
CT-996 would directly compete with oral products such as Rybelsus approved for patients with T2D and other oral pipeline developmental products from companies like Amgen, D&D Pharmatech, Eli Lilly, Hengrui, Pfizer, Sciwind Biosciences, Structure, and Viking.
CT-868 has limited competition for T1D as there are no currently approved products that would be direct competitors. However, there are two peripheral products, liraglutide (Novo Nordisk) and semaglutide (Novo Nordisk) approved for general weight loss, including both T1D and T2D patients. Other pipeline products targeting different aspects of T1D ranging from prevention to cell therapy from Cell Trans, Dompe, Provention Bio, and Vertex are mentioned, but they are not considered direct competitors for CT-868.
The above promising treatments, if approved, would face competition based on efficacy, safety, convenience, price, level of generic competition, and availability of reimbursement.
Moreover, the market opportunity for obesity treatments is expanded due to the strong linkage between obesity and T2D. This creates a potential for treatment options that can not only assist with weight management but also provide beneficial impacts on glycemic control and prevention of diabetes-related complications.
CT-388 could potentially fit into the treatment paradigm if the following criteria based on clinical studies are met:
Efficacy: CT-388 should demonstrate significant weight loss that is sustained over a longer-term period, as obesity requires chronic management. The drug shows promise with "statistically significant and dose-proportional weight loss" observed across cohorts and a notable average of 8.4% weight loss in the highest dose cohort at four weeks. If these results are replicated in larger studies and over longer periods, this could position CT-388 as a strong candidate for obesity treatment.
Safety and Tolerability: The available data suggest that CT-388 is generally well-tolerated, with the most common treatment-emergent adverse events (TEAEs) being GI-related, which are common to incretin therapies. No participants discontinued treatment due to TEAEs, which is encouraging for the medication's tolerability. It will be crucial for future studies to further elucidate the safety profile of CT-388, especially when administered at higher doses and over more extended periods.
Convenience: CT-388's pharmacokinetic (PK) profile supports once-weekly dosing, which offers a more convenient regimen compared to daily injections. This could improve patient adherence and satisfaction compared to existing therapies, making it an attractive option if efficacy and safety are confirmed.
Insulin Sensitivity: There was an observed improvement in HOMA-IR, indicating increased insulin sensitivity with CT-388 treatment. This effect could be particularly beneficial for patients with obesity who have or are at risk of developing T2D.
Comorbidities: The company suggests that future indications for CT-388 could include a range of obesity-related comorbid conditions such as nonalcoholic steatohepatitis (NASH), cardiovascular disease (CVD), heart failure, osteoarthritis, sleep apnea, and chronic kidney disease (CKD). This hints at the potential for CT-388 not just as a weight-loss medication but also as part of the treatment strategy for related diseases, further solidifying its place in the standard of care if it proves to be effective in these areas.
Ultimately, the success of CT-388 in the standard care of obesity will depend on robust data from clinical trials demonstrating a favorable balance of efficacy, safety, and tolerability over existing therapies. The ongoing and future development of CT-388 will reveal its viability as a treatment, and its positioning in the market will be determined by long-term outcomes and real-world effectiveness. If the promising trends noted in the early phases continue, CT-388 may well become an integral part of the standard of care for obesity.
Type 2 Diabetes
The market opportunity in Type 2 Diabetes (T2D) is significant due to its high prevalence, associated comorbidities, and substantial economic burden. With over 400 million people worldwide suffering from diabetes, the majority of whom have T2D, this condition not only impacts the health of individuals but also places a heavy strain on healthcare systems.Successful Drugs in the Indication:
- Metformin: As the standard first-line medication for T2D, metformin helps decrease hepatic glucose production and improve insulin sensitivity.
- Sulfonylureas: Glipizide and glyburide, among others, act by stimulating pancreatic beta-cells to release more insulin.
- Thiazolidinediones (TZDs): Pioglitazone and rosiglitazone work by improving insulin sensitivity.
- DPP-4 inhibitors: Sitagliptin and saxagliptin enhance the lifespan of endogenous incretins involved in insulin release.
- SGLT2 inhibitors: Empagliflozin and canagliflozin increase glucose excretion through the kidneys.
- GLP-1 receptor agonists: Liraglutide and semaglutide improve glycemic control by enhancing insulin secretion and suppressing appetite, with additional cardiovascular benefits.
- Insulin therapy: For patients needing more direct glycemic management, various forms of insulin are used, sometimes in conjunction with the above agents.
Standard of Care:
The standard care for T2D typically starts with lifestyle changes in conjunction with metformin therapy. For patients who do not achieve glycemic targets with metformin, additional oral agents, injectables, or insulin therapies may be layered on. The choice of additional therapies depends on various individual patient factors including cardiovascular health, kidney function, risk of hypoglycemia, and body weight.
Unmet Medical Need:
Despite numerous available treatments, there are still unmet medical needs within the T2D patient population, which present opportunities for new market entrants. These include:
- Need for Weight-Neutral or Weight-Reducing Therapies: Many diabetes medications can lead to weight gain, yet the majority of people with T2D are overweight or obese.
- Simplification of Treatment Regimens: More convenient dosing schedules, such as once-weekly injections, could improve patient adherence.
- Reduction in Treatment-Related Side Effects: Minimizing side effects, particularly hypoglycemia and gastrointestinal issues, is a priority.
- Cost-Effectiveness: More affordable medications could improve access to therapy for many patients.
- Cardiorenal Protection: There is a need for therapies that not only control blood sugar but also protect against the macrovascular and microvascular complications of diabetes.
- Personalized Treatment: As T2D is heterogeneous, there is a need for more personalized approaches to treatment considering the patient's specific pathophysiology and comorbidities.
- Minimizing Disease Progression and Complications: Treatments that can alter the course of T2D or reduce its complications are in high demand.
Given this market context, the opportunity for new therapeutic agents is considerable. Drugs that meet some or all these unmet needs have the potential for substantial clinical and commercial success. Evidence of improved glycemic control with fewer side effects, weight loss benefit, improved cardiovascular outcomes, and slower disease progression can differentiate a new drug in the T2D landscape. With an aging population and increasing rates of obesity worldwide, the prevalence of T2D is expected to continue to rise, further underscoring the growing market need for effective treatments.
The field of Type 2 Diabetes (T2D) treatment is continually advancing with several promising treatments currently under development that aim to address the limitations of existing therapies and fulfill the unmet medical needs. Below is a summary of some key areas of development:
GLP-1 Receptor Agonists: This class of drugs continues to be a focus of development due to its efficacy in both glycemic control and weight reduction. Examples include semaglutide with oral (Rybelsus) and injectable (Ozempic) formulations, and dulaglutide (Trulicity), both with favorable cardiovascular outcomes data. Newer agents are exploring higher efficacy and longer duration of action.
SGLT2 Inhibitors and Combinations: These drugs, which include empagliflozin (Jardiance) and canagliflozin (Invokana), have demonstrated cardiorenal protection benefits. Combination therapies that unify the benefits of SGLT2 inhibition with other glucose-lowering mechanisms (like GLP-1 agonism) are also in development to provide multifaceted treatment approaches.
Dual and Tri-Agonists: Molecules that simultaneously target multiple gut hormone receptors, such as GLP-1, GIP (Glucose-Dependent Insulinotropic Polypeptide), and glucagon receptors, are being developed. Tirzepatide (Mounjaro) is a dual GIP and GLP-1 receptor agonist showing promising results in weight loss and glycemic control, suggesting that multi-agonists may be advantageous in managing T2D.
Novel Insulin Formulations: New formulations of insulin that offer extended action or more physiologic profiles are being investigated to enhance patient convenience and adherence by reducing the frequency of injections and mimicking natural insulin release more closely.
Oral Insulins and Adjunct Therapies: Although challenging to develop, oral insulin formulations remain an area of interest due to the potential benefits in patient convenience and adherence. Additionally, adjunctive therapies that can be used alongside insulin to improve its efficacy or reduce its required dosages are under exploration.
Incretin Enhancers: Drugs that enhance the activity of incretins, such as DPP-4 inhibitors, are also being further developed and combined with other mechanisms to create synergistic effects.
Non-Insulin Injectable Therapies: Newer injectable therapies, including those not based on the incretin system, are in the pipeline, targeting different biological pathways involved in T2D pathogenesis.
Cellular and Gene Therapies: Revolutionary approaches, such as the use of gene therapy to restore insulin production or the transplantation of insulin-producing cells, are being researched, with some promising early results.
Precision Medicine Approaches: There's also an increasing emphasis on personalized treatments based on individual genetic, environmental, or lifestyle factors that contribute to their T2D.
The competitive landscape involves numerous established companies with significant resources and experience but also features smaller biotechnology firms that specialize in innovative approaches. These competitors are advancing treatments that could offer better control of blood glucose levels, reduced side effects, fewer injections, weight loss benefits, and improved cardiovascular outcomes.
Every new drug entering the T2D market will need to demonstrate clear benefits over existing therapies in terms of efficacy, safety, convenience, and cost-effectiveness. Additionally, as the understanding of T2D as a multifactorial and heterogeneous disease grows, the development of treatments that can be tailored to individual patient needs or that can slow the progression of the disease will be particularly valuable.
Finally, market access issues, such as drug pricing and reimbursement by insurers, will be crucial for the success of new T2D therapies. Drugs that can demonstrate long-term cost savings, for instance by reducing hospitalizations or complications related to diabetes, will be poised for favorable reception in the market.
The treatment landscape for Type 2 Diabetes (T2D) has grown significantly with the approval of several notable drugs that focus on different aspects of the disease pathophysiology. Here are some of the key medications, including recently approved branded drugs, used to treat T2D:
Notable Recently Approved Drugs:
Semaglutide (Oral, brand name Rybelsus): Approved in 2019, Rybelsus is the first oral formulation of a GLP-1 receptor agonist, offering an alternative to injections for patients with T2D.
Tirzepatide (brand name Mounjaro): Received FDA approval in May 2022, this is a dual GIP and GLP-1 receptor agonist indicating a novel class of drugs offering significant improvements in glycemic control and weight reduction.
Ertugliflozin (brand name Steglatro): Approved in 2017, this is an SGLT2 inhibitor that, apart from lowering blood sugar levels, has been evaluated for its cardiovascular benefits.
Dulaglutide (Trulicity): Though not recently approved as it entered the market in 2014, it's worth mentioning due to a once-weekly injection schedule and its cardiovascular benefits demonstrated in later trials.
Icosapent ethyl (brand name Vascepa): While not a direct glucose-lowering medication, this purified fish oil derivative was approved in December 2019 for cardiovascular risk reduction in adults with T2D and established cardiovascular disease, addressing a significant comorbidity.
The drugs mentioned above have significantly impacted the management of T2D by improving glycemic control, providing additional cardiovascular benefits, and offering more patient-friendly dosing options. The continued innovation in T2D medication aims to further address the unmet needs in the diabetic population, particularly around ease of use, efficacy, and overall benefits beyond glycemic control.
Given the well-established role of the GLP-1 pathway in T2D management, CT-388 could potentially fit within the current standard of care in several ways:
Weight Loss: CT-388 has demonstrated significant, rapid, and dose-proportional weight loss in the trial subjects, which is a key therapeutic goal in both obesity and T2D management. Reducing obesity often leads to improved glycemic control in people with T2D, positioning CT-388 as both an obesity and diabetes management option.
Insulin Sensitivity: The drug has shown a notable decrease in HOMA-IR levels, indicating improved insulin sensitivity. As insulin resistance is a hallmark of T2D, improving this parameter could translate into better overall glycaemic control for patients.
Once-Weekly Dosing: The pharmacokinetics of CT-388 supports once-weekly dosing, making it a convenient option compared to daily injections. This may improve adherence to the treatment regimen, as seen with other once-weekly GLP-1 receptor agonists such as semaglutide (Ozempic) and dulaglutide (Trulicity).
Safety Profile: The tolerability of CT-388 appears to be favorable, with no treatment discontinuations reported due to adverse events and most side effects being mild to moderate in severity. A tolerable side effect profile is essential for long-term management strategies in chronic diseases like T2D.
Potential for Broader Application: CT-388 could have a broad impact by potentially improving a range of obesity-associated comorbidities, which often accompany T2D, such as nonalcoholic steatohepatitis (NASH), cardiovascular disease (CVD), and chronic kidney disease (CKD). Medications that address multiple aspects of metabolic syndrome are increasingly being recognized as valuable.
Competitive Landscape: If approved, CT-388 would likely join other GLP-1 receptor agonists and various combination therapies on the market. Its potential advantages in efficacy, safety profile, and once-weekly dosing could make it a strong candidate in the competitive T2D treatment market, assuming its benefits are further substantiated in upcoming trials.
However, it is important to note that although the data so far is promising, the success of CT-388 in the T2D market will depend on continued positive results from ongoing and subsequent clinical trials (such as Phase 2 and 3 trials). These trials will need to demonstrate sustained efficacy, long-term safety, and improved patient outcomes. Additionally, real-world evidence post-commercialization would be important to establish the drug's true clinical and cost-effectiveness relative to existing treatments.
Revenue build and model
Obesity
Given the complexity of obesity and its relationship to Type 2 Diabetes (T2D), as well as the existing treatments and research landscape, creating a hypothetical revenue build for CT-388 involves a number of assumptions and estimates that require careful consideration. Here is a framework for estimating potential revenue by considering different aspects relevant to the treatment's possible commercialization:
Hypothetical Revenue Build for CT-388
- Target Population Estimation:
- Prevalence: Building off the statistic of more than 750 million people affected by obesity worldwide.
- Accessible Population: Estimate the percentage of this population that CT-388 could realistically reach, considering geographic and healthcare access limitations.
- Market Penetration:
- Awareness and Adoption Rates: Considering efforts in marketing, education, and medical community outreach, estimate the potential adoption rate among healthcare providers and patients.
- Competitive Differentiation: Positioning of CT-388 against existing alternatives based on efficacy, safety, ease of use (once-weekly dosing), and potential to treat related conditions.
- Pricing Strategy:
- Determine a plausible pricing range for CT-388 based on comparative drugs and the value proposition of CT-388, noting any premium attributable to unique benefits.
- Consider potential variations in pricing across different markets (e.g., US vs EU vs emerging markets).
- Insurance Coverage and Reimbursement Scenarios:
- Private Insurance: Estimate the percentage of patients whose insurance would cover CT-388.
- Medicare/Medicaid & International Health Systems: Evaluate the likelihood and scope of coverage by public health systems.
- Duration of Therapy:
- Based on clinical data indicating when significant weight loss is achieved and considering obesity as a chronic condition, determine the average treatment duration.
- Assume treatment duration variations for responders vs non-responders, and factor in possible repeat courses.
- Patient Drop-off Rates:
- Based on tolerability data and general trends for chronic medications, estimate attrition rates year over year.
- Patient Compliance Estimates:
- Use conservatively adjusted estimates given the likely drop in compliance over time for chronic therapies.
- Sales and Distribution Plan:
- Channels: Direct sales to healthcare providers, online pharmacies, traditional pharmacies.
- Partnerships: Evaluate potential partnerships or licensing agreements that could impact revenue.
- Market Access Programs:
- Patient Assistance Programs: Factor in potential discounts or free product provisions for eligible patients which can influence overall revenue.
- Pay-for-Performance / Outcome-Based Reimbursement: Consider revenue implications of reimbursement agreements tied to patient outcomes.
- Scenario Analysis:
- Create optimistic, realistic, and pessimistic scenarios for all estimates to provide a revenue projection range.
- Update scenarios as new data emerges from ongoing and future trials.
Placeholder Estimates Example:
- Accessible Population: 100 million (adjust for target markets)
- Market Penetration Year 1: 0.5%, increasing to 3% by Year 5.
- Pricing: $600/month in the US with 50% gross-to-net adjustment; for other markets, adjust based on purchasing power parity and health system negotiations.
- Insurance Coverage: 70% of patients in Year 1, increasing based on health outcomes data supporting coverage.
- Duration of Therapy: 6 months average for initial treatment, with repeat or chronic use for 30% of patients.
- Patient Drop-off Rates: 15% annually.
- Patient Compliance: 80% consistently taking medication as prescribed.
These calculations should be revisited and refined as more specific data about CT-388 and the market dynamics comes to light, especially post-results from Phase 2 and 3 clinical trials. Additionally, regulatory approval pathways, competitor launches, advancements in obesity treatment paradigms, and changing healthcare policies would affect the above estimates significantly. A collaborative approach with stakeholders in the healthcare, financial, and patient advocacy sectors would be essential to maximize the potential of CT-388 for the benefit of patients and to realize appropriate revenue streams.
When considering the progression of a GLP-1/GIP agonist from Phase 2 to Phase 3 in the context of metabolic disorders, it's important to factor in the demonstrated clinical effectiveness and safety of GLP-1 agonists. This class of drugs has shown significant promise in treating metabolic disorders, which could potentially increase the probability of success (PoS) estimates compared to the industry standard.
The industry standard Phase 2 to Phase 3 progression rate for metabolic disorders is 45%, and the Phase 3 success rate is 63.6%. However, for a GLP-1/GIP agonist with positive preclinical and Phase 1 data, these rates might be adjusted upwards due to:
- Established Efficacy and Safety Profile of GLP-1 Agonists: The success of GLP-1 agonists in clinical trials and practice would support a higher confidence in the progression and success of similar agents.
- Synergistic Potential of GLP-1/GIP Combination: The combination of GLP-1 with GIP (Glucose-Dependent Insulinotropic Polypeptide) may offer enhanced efficacy in metabolic regulation, potentially increasing the likelihood of clinical success.
- Positive Preclinical and Phase 1 Data: Early-stage positive results can be indicative of future success, although it's important to remain cautious as many compounds fail in later stages despite early promise.
Given these factors, the Phase 2 to Phase 3 progression rate could be conservatively adjusted to a range slightly higher than the industry standard, perhaps around 50-60%. Similarly, the Phase 3 success rate might be adjusted upwards, potentially to a range of 65-75%, reflecting the positive data and established class efficacy.
It's important to note that these are speculative adjustments and actual success rates can vary based on numerous factors including the specific drug profile, trial design, patient populations, regulatory environment, and more. Therefore, while the historical success of GLP-1 agonists suggests a higher PoS, each new drug candidate must be evaluated on its own merits.
Type 2 Diabetes
Creating a hypothetical revenue build for CT-388 in its potential use for Type 2 Diabetes (T2D) treatment involves several considerations based on the available information. Here are possible estimates for each of the items mentioned, which should be considered placeholders and subject to change based on further clinical data and market dynamics:- Target Population Estimation:
- Global Prevalence of T2D: 400 million
- Marketable Population: Considering demographic distributions and market accessibility, target 10% of this population, resulting in 40 million potential users.
- Market Penetration:
- Project an increase to 2% penetration over five years, reaching 800,000 users.
- Insurance Coverage:
- Initial Coverage Rate: Assume 75% of patients will have insurance coverage, potentially increasing to 85% over five years.
- Duration of Therapy:
- Average Treatment Duration: Assume an average patient will be on CT-388 for two years, accounting for drop-offs and switches.
- Patient Drop-off Rates:
- Annual Attrition: Estimate a 10% annual discontinuation rate due to factors like side effects or switching treatments.
- Treatment Adherence:
- Adherence Rate: Assume an 80% adherence rate to weekly doses, adjusting for potential long-term decreases.
The figures given are entirely hypothetical and are not based on current market data or actual clinical trial outcomes for CT-388. In practice, constructing a revenue forecast would be an iterative process with inputs from market research, clinical development progress, and dynamic market conditions, involving many stakeholders such as finance experts, market analysts, and health economists.
CT-868
Scientific thesis
The therapeutic rationale for a GLP-1/GIP receptor agonist in Type 1 Diabetes (T1D) with overweight or obesity is multifaceted and grounded upon the effects of GLP-1 and GIP on glucose homeostasis, body weight, and overall metabolic health. CT-868 has been intentionally designed to act on both GLP-1 and GIP receptors with a bias towards the activation of the cAMP pathway without promoting ß-arrestin recruitment. Here are the key reasons for using a dual GLP-1/GIP receptor agonist in such a scenario:
GLP-1 Receptor Activation: The GLP-1 receptor is involved in several key metabolic processes. When activated, it enhances insulin secretion (in a glucose-dependent manner), suppresses glucagon release, slows gastric emptying, and directly affect neuronal pathways to reduce appetite. This can lead to improved glycemic control and weight loss, and has been shown to reduce the risk of hypoglycemia when compared to insulin alone. Clinical trials with existing GLP-1 receptor agonists have borne out these benefits, demonstrating significant reductions in HbA1c, weight, and often insulin requirements.
GIP Receptor Activation: GIP is another incretin hormone that plays a role in carbohydrate metabolism, enhancing insulin secretion in response to meals. Although it has not been as extensively targeted in therapeutics as GLP-1, there is evidence suggesting that GIP can also contribute to lowered blood glucose levels and improved insulin sensitivity, and potentially achieve insulin-independent glucose clearance.
Biased Signaling: CT-868 has been designed as a biased agonist, which favors the activation of the cAMP pathway while not recruiting ß-arrestin to the receptors. This is considered an advantage because ß-arrestin can prompt receptor internalization and desensitization, leading to diminished therapeutic effects over time. By not recruiting ß-arrestin, CT-868 might lead to prolonged and more sustained receptor activation without these negative feedback mechanisms, which could translate into prolonged glucose lowering and weight loss effects along with a potentially better side effect profile.
Synergistic Effect: The combined action on both GLP-1 and GIP receptors is speculated to produce a synergistic effect that could result in greater glycemic control and weight reduction than targeting either receptor alone, based on the positive clinical trial outcomes noted with the first approved dual GLP-1/GIP receptor agonist, tirzepatide.
Clinical Trials: The data from the Phase 1 and Phase 2 clinical trials with CT-868 demonstrate significant improvements in HbA1c and glucose homeostasis with concurrent lower insulin levels, suggesting that CT-868 has the potential to improve blood sugar control while also decreasing the amount of insulin T1D patients might need. This can have a positive impact on weight management, given the weight-gain-associated side effect of insulin therapy.
Adjunct to Insulin Therapy: Using CT-868 as an adjunct to insulin holds promise for improving glycemic control while potentially reducing the side effects associated with insulin therapy alone (such as weight gain and the risk of hypoglycemia) and providing additional benefits like weight loss.
Safety Profile and Tolerability: Biased signaling may also improve the tolerability of the therapy. It has the potential to offer a more favorable side effect profile, reducing the gastrointestinal side effects commonly seen with incretin-based therapies.
Research and Development Approach: The development of CT-868 uses an advanced platform, Chemotype Evolution, which allows for strategic iterative compound optimization focusing on potency, selectivity, bioavailability, half-life, and biased-signaling properties, to improve the therapeutic index of the candidate.
In summary, based on preclinical and clinical studies, the addition of a dual GLP-1/GIP receptor agonist like CT-868 to insulin therapy for the treatment of T1D in patients with overweight or obesity could offer significant benefits in terms of improved glycemic control, reduced insulin doses, and weight loss, while also likely having a favorable safety and tolerability profile due to its intentional signaling bias. The ongoing Phase 2 proof-of-concept clinical trial and the upcoming data release will be critical in evaluating and confirming these potential benefits and therapeutic rationale.
The science behind GLP-1 and GIP receptor agonists is relatively well established, particularly the physiological roles of GLP-1 and GIP in glucose homeostasis and their potential as targets for diabetes treatment. However, as with all emerging treatments, there are aspects that are still subject to continuing research, uncertainty, and scientific debate. Here are some points on the state of the science and areas of ongoing inquiry:
GLP-1 Receptor Agonists: The use of GLP-1 receptor agonists in managing type 2 diabetes is well-established and supported by robust clinical evidence. These agents have been shown to improve glycemic control and promote weight loss in this patient population.
GIP Receptor Effects: The therapeutic role of GIP is less well understood compared to GLP-1. There is emerging evidence suggesting that GIP receptor activation has beneficial effects on glucose homeostasis, though historically it was challenging to target due to the complex nature of GIP action in type 2 diabetes. The recent approval of tirzepatide, a dual GLP-1/GIP receptor agonist, indicates a growing recognition of the potential therapeutic benefit of GIP receptor activation.
Dual GLP-1/GIP Receptor Agonists: While clinical evidence is growing for dual-acting therapies, it is a more recent area of development compared to GLP-1 monotherapy. Tirzepatide set a precedence in demonstrating the clinical efficacy of dual agonism in type 2 diabetes patients, but there is still much to learn about how these therapies perform in type 1 diabetes, and how they affect weight, glycemic control, insulin doses, and overall metabolism when used as an adjunct to insulin.
Biased Signaling: The concept of biased signaling is at the frontier of pharmacology research and offers promising strategies for drug development. While preclinical and early clinical studies suggest potential benefits, the long-term clinical implications of this approach remain an area of active investigation. Further clinical trials are necessary to conclusively determine the relative merits and any unforeseen consequences of biased agonism in the treatment of diseases, including diabetes.
Uncertainty in T1D Treatment: While CT-868 has demonstrated encouraging results in preclinical studies and early-phase clinical trials in populations with T2D and overweight or obesity, transferring these findings to T1D involves a different pathophysiological context. The inherent autoimmune destruction of beta cells in T1D means that exogenous insulin is always necessary, and how these incretin-based therapies modify the T1D disease process is less clear.
Safety and Side Effects: Although biased signaling might theoretically improve tolerability, the actual side effect profile in diverse patient populations over long-term use requires extensive clinical evaluation. Gastrointestinal (GI) side effects are a common limitation of incretin-based therapies, and while biased agonism may reduce these, determining this requires rigorous clinical trial data.
Overall Level of Evidence: The science underpinning the use of GLP-1 receptor agonists in diabetes management is robust, but the evidence supporting the combined GLP-1/GIP approach and the concept of biased signaling is less extensive and is derived primarily from preclinical studies, early-phase clinical trials, and newer substantial trials (like those for tirzepatide).
While the majority of the literature surrounding GLP-1/GIP receptor agonists has focused on Type 2 Diabetes (T2D), there is a growing body of evidence exploring their potential in Type 1 Diabetes (T1D), particularly in individuals with overweight or obesity. Here are some findings from the literature that support the investigation into GLP-1/GIP's role in T1D:
- GLP-1 Receptor Agonists in T1D:
- A review article by Varanasi et al. (Diabetes, Obesity and Metabolism, 2011) discusses the potential of GLP-1 receptor agonists in managing T1D. It suggests that GLP-1 agonists can help enhance endogenous insulin secretion (if residual beta-cell function is present), suppress glucagon production, slow gastric emptying, and reduce appetite, which can be beneficial for weight management in T1D patients who are overweight or obese.
Clinical trials, such as one published by Kielgast et al. (The Lancet, 2010), have demonstrated that adjunctive therapy with GLP-1 receptor agonists in T1D patients can lead to improved postprandial glucose control and reduced insulin requirements, possibly due to the extra-glycemic effects like gastric emptying and satiety enhancement.
GIP and Dual Agonism:
- Research on the GIP receptor's role in glucose control for T1D is not as extensive. However, GIP is known to synergize with insulin to promote glucose disposal, suggesting that GIP receptor activation could help lower glucose levels and modulate insulin dosage in T1D.
The exploration of dual GLP-1/GIP receptor agonists, such as the above-mentioned tirzepatide, is mostly in the context of T2D. Studies show that these dual-acting agents result in significant improvement in glycemic control and promote weight loss. These data provide a rationale for the exploration of dual agonists in the context of T1D, especially considering the benefits of weight reduction and potential for improved control over blood sugar levels.
Obesity and T1D: Overweight and obesity are increasingly recognized as significant issues in T1D management as they can exacerbate insulin resistance and cardiovascular risk. A publication by Purnell et al. (Diabetes Care, 2013) notes that obesity in T1D is associated with greater total and abdominal fat mass, and highlights the need for therapeutic strategies addressing weight management in T1D patients.
Much of the clinical evidence for GLP-1/GIP receptor agonists' effects in T1D as adjuncts to insulin therapy remains preliminary. It largely derives from small studies or extrapolations from T2D data and, as such, larger and more definitive clinical trials were eagerly awaited. Since clinical evidence and guidelines are continually evolving, it is important to consult the latest research literature and clinical trial results for the most current understanding and treatment recommendations for T1D with concurrent overweight or obesity.
The evidence base supporting the therapeutic rationale for using GLP-1/GIP receptor agonists in Type 1 Diabetes (T1D) with overweight or obesity has both strengths and weaknesses. Below is an analysis of these:
Strengths:
Physiological Actions of GLP-1 and GIP: There is a strong understanding of the mechanistic role of GLP-1 and GIP in glucose homeostasis. GLP-1 is known to enhance insulin secretion, inhibit glucagon release, slow gastric emptying, and promote satiety—all of which contribute to better glycemic control and may support body weight regulation.
Evidence from Type 2 Diabetes (T2D): The efficacy of GLP-1 receptor agonists in controlling hyperglycemia and promoting weight loss is well documented in T2D. This provides a solid rationale for exploring their use in T1D, especially since overweight and obesity are common concerns in both types of diabetes.
Preclinical Data: Animal studies often provide initial insights into the pharmacological effects of new treatments. The benefits of GLP-1 and GIP receptor agonists have been established in preclinical models, providing a foundation for clinical research.
Early Clinical Trials: Some small and early-stage clinical trials and pilot studies have suggested that GLP-1 receptor agonists might improve glycemic control and reduce insulin doses in T1D patients, adding a layer of support for the proposed therapeutic approaches.
Incretin Therapeutic Agents' Track Record: Existing incretin-based therapies, such as GLP-1 receptor agonists like liraglutide and semaglutide, have already been used in the clinic, providing real-world data on their efficacy and safety.
Weaknesses:
Limited Direct Evidence for T1D: While the use of GLP-1 receptor agonists in T2D is well supported, there is a paucity of large-scale, long-term clinical trials examining their use as adjuncts to insulin in T1D, particularly for those with overweight or obesity.
Shortcomings of Animal Models: While preclinical data can be suggestive, they do not always accurately predict clinical outcomes in humans due to differences in physiology, disease progression, and complex metabolic interactions that cannot be fully replicated in animal models.
Lack of Long-Term Safety Data: Since many of the studies are short-term or the treatments are innovative (like dual GLP-1/GIP agonism), there is limited long-term safety data available, which is crucial to identifying potential chronic side effects or complications.
Methodological Limitations in Clinical Trials: Some studies may have small sample sizes, short durations, or limited demographic representation. Additionally, the lack of comparator arms in some studies makes it challenging to ascertain relative efficacy and safety.
Extrapolation from T2D Treatments: T1D has a different pathophysiology than T2D, including autoimmune beta-cell destruction. Therefore, treatments effective in T2D may not have the same effects or may need to be used differently in T1D.
Weight Management Complexity: While GLP-1 receptor agonists have shown promise in contributing to weight loss in T2D, weight management in T1D is multifactorial and not solely dependent on the actions of these agents.
Bias Signaling Concept: The idea of biased signaling providing clinical benefits is innovative but still relatively novel in endocrinology. More research is needed to confirm its potential advantages over traditional receptor activation approaches.
In conclusion, while there is a sound scientific basis for the potential benefits of GLP-1/GIP receptor agonists in T1D with overweight or obesity, the clinical evidence is still evolving, and larger, more rigorous clinical trials are necessary to establish their efficacy, optimal usage protocols, and safety in this specific patient population.
Clinical trial overview
The company is studying the drug in a Phase 1 clinical study titled "A Randomized, Double-Blind, Placebo and Comparator-Controlled Crossover Study to Assess the Effects of CT-868 Treatment on Glucose Homeostasis in Participants With Type 1 Diabetes".
Design:
- Type: Interventional - Phase 1 Clinical Trial
- Enrollment: Estimated 24 participants
- Start/Completion:
- Actual Start: March 21, 2023
- Estimated Primary Completion: February 2024
- Estimated Study Completion: February 2024
Methods:
- Participants: People with Type 1 Diabetes and overweight or obesity
- Interventions:
- Experimental: CT-868 via subcutaneous (SC) injection
- Placebo Comparator: Placebo via SC injection
- Active Comparator: Victoza (Liraglutide) via SC injection
- Allocation: Randomized
- Intervention Model: Crossover Assignment
- Masking: Quadruple - Participant, Care Provider, Investigator, Outcomes Assessor
Outcome Measures:
- Primary: Assess AUC in glucose metabolism during a Mixed Meal Tolerance Test (MMTT) - measured from baseline up to 4 days.
- Secondary: Changes in continuous glucose monitoring (CGM) measures, Area under the acetaminophen concentration-time curve (AUC), from 0 to 300 minutes.
Study Critique:
- Strengths:
- The crossover design allows for each participant to serve as their own control, which can increase the statistical power.
The study is double-blind and placebo-controlled, which minimizes bias in treatment effects.
Critiques:
- With a small estimated enrollment of just 24 participants, the study may lack statistical power and may not be generalizable to all patients with Type 1 Diabetes.
- The study duration for each intervention seems short (up to 4 days), which may not be sufficient to evaluate long-term effects or side effects of the drug.
- The inclusion of a marketed drug (Victoza) as an active comparator is a good practice, but differences in mechanisms of action could confound the results unless clearly accounted for.
Operational or Technical Challenges:
- Patient Recruitment: Enrolling a specific subset of people with Type 1 Diabetes who are also overweight or obese might pose challenges and potentially extend recruitment periods.
- Adherence to Protocol: Ensuring that each participant adheres to the rigorous treatment and testing schedule during the crossover design could be challenging.
- CGM Data Integrity: Continuous glucose monitoring systems can sometimes produce inaccurate readings which need to be validated or addressed using calibrations.
- Drug Preparation & Delivery: Preparing and administering the SC injections while maintaining the double-blind nature of the study requires meticulous planning and training of the care providers.
- Acetaminophen Interference: The use of acetaminophen as a measure of drug absorption or gastric emptying needs to be carefully controlled, as it may interfere with CGM readings or have other effects on participants' health.
Overall, while the design of this CT-868 study is quite standard for early-phase clinical trials, careful attention should be given to data collection, participant adherence, and to the interpretation of the results, keeping in mind its limited sample size and study duration.
The study aims to evaluate the effects of CT-868 on glucose homeostasis in participants with Type 1 Diabetes Mellitus (T1DM) with overweight or obesity, which is a reasonably specific patient population that could potentially benefit from a new therapeutic option. This proof-of-concept study seems to be well designed to establish initial efficacy and pharmacodynamics of CT-868.
Appropriateness of Endpoints:
Primary Endpoint: Assessing the area under the curve (AUC) in glucose metabolism during a Mixed Meal Tolerance Test (MMTT) is an appropriate primary endpoint for this proof-of-concept study. MMTT is a standard test to assess postprandial glucose control and insulin secretion, which are critical aspects of glucose homeostasis impacted by T1DM.
Secondary Endpoints: Measuring changes in continuous glucose monitoring (CGM) data can provide insights into daily glucose profiles, which are important for reflecting overall glycemic control outside the clinical setting. Assessing the area under the acetaminophen concentration-time curve (AUC) provides an indirect measure of gastric emptying, which can influence postprandial glucose levels.
Inclusion Criteria:
The inclusion criteria are chosen to define a homogenous study population affected by T1DM and carrying additional metabolic concerns due to being overweight or obese (BMI 25.0 - 35.0). This demographic may have different responses to treatments compared to non-overweight individuals.
- Age 18-65 ensures the study involves adult and older adult populations but excludes pediatric and older populations that might have different pharmacodynamic responses or a more complex medical history.
- Diagnosis of T1DM for at least 3 years and management with an insulin pump or MDI for at least 3 months ensure that participants have an established T1DM condition and that their diabetes management is stable.
Exclusion Criteria:
- Excluding participants with significant medical history, uncontrolled diabetes, history of surgical treatment for weight loss, or history of malignancy is intended to reduce variability that could obscure the effects of CT-868 and also to protect participants who might be at higher risk of adverse effects.
Reproducibility Challenges:
Inclusion / Exclusion Criteria: A narrowly defined population (BMI 25.0-35.0) might limit enrolment and the generalizability of the study findings. Participants with a BMI just outside this range, whether lower or higher, may still benefit from CT-868, but their response could be different, affecting reproducibility in the wider T1DM population. The demand for stable insulin therapy via pump or MDI could exclude those who are not well-controlled on these therapies or who manage their diabetes by other means, again affecting the external validity and reproducibility of the study.
This study appears capable of providing valuable data on the safety and initial effectiveness of CT-868 in a specific subset of T1DM patients. The careful selection of endpoints, inclusion, and exclusion criteria shows the intention to minimize variability and potential confounders. However, the transferability of results to the broader T1DM patient population or to subsequent phases of clinical trials may face challenges due to the relatively strict criteria. It will be crucial for further clinical trials to include a more diverse patient population to confirm the reproducibility and generalize the study results.
Clinical trial results
Here is a summary of the clinical data supporting CT-868:
Safety and Tolerability: The Phase 1 trial in participants with overweight and obesity showed that CT-868 doses up to 11 mg in single ascending dose (SAD) and 5 mg in multiple ascending dose (MAD) portions were safe and tolerable, supporting its advancement into Phase 2 trials.
Efficacy: In the Phase 2 trial for Type 2 Diabetes (T2D) participants with overweight or obesity, there was a significant placebo-adjusted decrease in HbA1c of 2.3% at the 26-week mark with 4 mg CT-868, indicating a substantial impact on glycemic control. Although the indication for T1D is different, these results lend confidence for its application in T1D due to the similarities in the metabolic outcome that CT-868 influences.
Treatment-Emergent Adverse Events (TEAEs): TEAEs were common but primarily mild across all CT-868 treatment groups and placebo, with most participants experiencing at least one TEAE and few reporting serious TEAEs. There were no discontinuations due to TEAEs in the CT-868 groups.
Gastrointestinal (GI) TEAEs: Gastrointestinal disorders were among the more common TEAEs, with the highest incidence overall in the CT-868 4 mg dose group. Notable were increased constipation and nausea in the 4 mg group, and an increase in diarrhea in both the 1.75 mg and 4 mg groups, compared to placebo.
Comparison with Other Treatments: In a separate Phase 1 trial for T2D participants, CT-868 demonstrated lower plasma glucose and lower insulin levels compared to liraglutide (an unbiased GLP-1 receptor agonist) and placebo, suggesting a potential unique mechanism of action beneficial for glucose regulation, without being dependent on weight loss.
Mechanism of Action in T1D: Based on preliminary data, CT-868 may have a GIP-mediated effect on insulin-independent glucose disposal which could translate into benefits for T1D patients.
Ongoing and Future Development: A Phase 1 trial is ongoing to assess glucose homeostasis in T1D participants, with results expected in the first half of 2024. Additionally, a Phase 2 proof-of-concept trial has commenced for T1D participants with overweight or obesity to assess the percentage change in HbA1c among other endpoints, with results expected in the second half of 2024. Plans are also in place to evaluate CT-868's effects in other T1D patient populations.
In summary, CT-868 has shown promise in early-phase trials in T2D, which supports its continued development for T1D in participants with either overweight or obesity. The upcoming Phase 2 trial data will be critical for further assessment of its efficacy and safety within the T1D indication.
Market overview
Type 1 Diabetes with overweight or obesity
The market opportunity in Type 1 Diabetes (T1D) is significant, specifically among individuals who are also overweight or obese. This population presents unique challenges in disease management due to the compounded effects of having T1D and excess weight.
Market Opportunity:
Growing Prevalence of Obesity in T1D: As the rates of obesity rise globally, there is also an increase in the number of individuals with T1D who are overweight or obese. This trend suggests a growing market for therapies that can effectively manage both T1D and obesity.
Complex Disease Management Needs: Patients with T1D require strict blood glucose control, which can be complicated by excess weight. Obesity may worsen T1D by increasing insulin resistance and the required insulin doses, leading to a vicious cycle of weight gain and elevated insulin needs. Therapies that can break this cycle could significantly improve patient outcomes.
Unmet Medical Need: Despite advancements in diabetes care, less than one-third of T1D patients consistently achieve target blood glucose levels. This indicates a substantial unmet medical need for better management options, particularly for those with coexistent obesity.
Other Drugs and Standard of Care:
Insulin Therapy: The mainstay of T1D management is exogenous insulin administration through injections or pumps. However, insulin therapy can contribute to weight gain, highlighting the need for additional therapeutic approaches that can aid in weight management without compromising glycemic control.
Weight Management Medications: While various drugs are approved for weight loss in the general population, their safety and efficacy specifically in T1D patients need to be established.
Existing Therapies and Analogues: Successful drugs in the field of diabetes (primarily T2D), such as GLP-1 receptor agonists (e.g., semaglutide) and SGLT2 inhibitors (e.g., empagliflozin), have shown benefits in both lowering blood glucose and aiding weight loss. Investigating whether such drugs could also benefit T1D patients with obesity could be promising.
Economic Impact:
Reduction in Healthcare Costs: An effective treatment that addresses both T1D and obesity has the potential to reduce the substantial healthcare costs associated with these conditions, particularly in terms of preventing or mitigating diabetes-related complications.
Insurance and Payer Benefits: Insurers and payers may be interested in treatments that can reduce long-term expenses by improving health outcomes and reducing the need for acute care and interventions.
Summary:
An effective therapeutic intervention for T1D patients with overweight or obesity represents a significant market opportunity due to the high and growing prevalence of these coexisting conditions, the complexity of their management, and the substantial unmet medical need. Any company that can deliver a treatment that is safe, effective, and improves quality of life for this patient population has the potential to make a major impact on the market. Given the economic burden of diabetes and its complications, successful entry into this space could also alleviate financial strain on healthcare systems and provide cost savings for payers and patients alike.
CT-868 appears to be a promising investigational drug that might offer a new approach to the treatment of patients with Type 1 Diabetes (T1D) who are overweight or obese. The inclusion of this therapy into the current standard of care may depend on several factors as it continues through clinical trials:
Efficacy: According to the Phase 2 clinical trial data, CT-868 shows a substantial placebo-adjusted decrease in HbA1c levels in participants with Type 2 Diabetes (T2D) and overweight or obesity. If similar results translate to patients with T1D, this could indicate that CT-868 is effective in improving glycemic control when used alongside insulin therapy.
Tolerability: The safety profile from the trial suggests that CT-868 is generally well-tolerated, with most treatment-emergent adverse events (TEAEs) being mild and comparable to placebo, and low discontinuation rates. GI side effects such as nausea and diarrhea seem to be the most notable, especially at higher doses, but these symptoms are also common with other incretin-based therapies on the market.
Weight Management: The fact that CT-868 leads to lower plasma glucose and insulin levels independent of weight loss is intriguing, as it may suggest additional metabolic benefits that could be particularly valuable for T1D patients struggling with overweight or obesity.
Mechanism of Action: The separate mechanism of action (MOA) study showing lower plasma glucose and insulin levels with CT-868 compared to liraglutide or placebo could be indicative of enhanced glucose disposal or decreased endogenous glucose production. This might hint at an advantage over 'unbiased' GLP-1 receptor agonists by potentially addressing the issue of insulin resistance, which is a significant concern for overweight or obese individuals.
Given these factors, CT-868 could potentially fit into the standard of care for T1D with overweight or obesity by providing not just another tool to better manage blood glucose levels but also possibly assisting with weight management. The ongoing and future clinical trials in T1D patients will be crucial for determining where CT-868 stands in comparison to existing treatments, both in terms of minimizing the risk of complications associated with T1D and addressing the specific needs of the overweight or obese T1D population.
If future trials can demonstrate consistent efficacy and tolerability, along with a favorable impact on weight, CT-868 could offer a significant advancement in T1D care. It could become part of a multi-pronged therapeutic approach that includes insulin for essential glycemic control, combined with treatment options like CT-868 to improve metabolic outcomes and mitigate the risk of obesity-related complications.
The drug development and approval process will also consider the cost-effectiveness of CT-868, patient preference for administration route, the need for titration schedules, and the overall risk-benefit profile. Should the data continue to support its use, CT-868 may be well-positioned to improve the standard of care for this patient population.
Revenue build and model
Type 1 Diabetes with overweight or obesity
Let's outline a hypothetical revenue build for CT-868 in Type 1 Diabetes (T1D) patients with overweight or obesity. Please be aware that the actual values will need to be determined by in-depth market research, data analysis, and clinical trial outcomes. The figures here are placeholders and illustrative examples to show what kinds of data will be needed for the revenue model.
Preliminary Assumptions for Revenue Estimation:
- Target Population Size: Determine the number of T1D patients within the market who are also classified as overweight or obese.
- Market Penetration: Estimate the percentage of the target population that CT-868 can realistically reach, considering competition, drug efficacy, convenience, and physician/patient acceptance.
- Treatment Cost: Define the annual or monthly cost of the therapy regimen for CT-868 based on pricing strategies, manufacturing costs, and market standards.
- Duration of Therapy: Estimate average treatment length for a patient on CT-868.
- Access to Treatment: Consider the percentage of the target population with access to the healthcare system and the ability to be prescribed CT-868.
- Insurance Coverage: Assess payer landscape to estimate what percentage of patients will have the drug covered by insurance and at what reimbursement rate.
Placeholder Estimates for Illustrative Revenue Build:
Target Population Size: Let's assume there are 1 million T1D patients with overweight or obesity in the target market.
Market Penetration: Hypothetically, CT-868 gains a market share of 10% after 5 years of marketing.
Treatment Cost: For example, the drug is priced at $15,000 for an annual treatment course per patient, with a 50% gross-to-net adjustment.
Duration of Therapy: The average duration of therapy is estimated at 2 years per patient, considering management of T1D is typically long-term.
Access to Treatment: Let's posit that 60% of the target population has access to CT-868 owing to various healthcare system factors.
Insurance Coverage: Assume that 80% of the patients will have CT-868 partially covered by insurance, with an average co-pay of $3,000 annually.
Please note that the above model is a simplified example. A real-world revenue model would include additional complexities such as tiered insurance reimbursements, geographical pricing variations, the impact of competition over time, potential discounts, patient assistance programs, as well as market and treatment dynamics influencing therapy initiation and discontinuation rates. Moreover, it is essential to update the model with real data as it becomes available, especially post-marketing data that can drastically affect assumptions on market penetration and duration of therapy.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
CT-996
Scientific thesis
The glucagon-like peptide-1 (GLP-1) receptor agonists represent a class of therapeutics that have become increasingly significant in the management of obesity and T2D due to their multifaceted mechanisms of action. The therapeutic rationale for using GLP-1 receptor agonists in these conditions revolves around their ability to increase insulin secretion, decrease glucagon secretion, slow gastric emptying, and reduce appetite and food intake, all of which contribute to improved glycemic control and weight loss.
Enhanced Insulin Secretion: GLP-1 receptor activation leads to increased insulin secretion from pancreatic beta-cells in a glucose-dependent manner, meaning it promotes insulin release when glucose levels are high, reducing the risk of hypoglycemia.
Decreased Glucagon Secretion: By inhibiting glucagon secretion from pancreatic alpha-cells, GLP-1 receptor agonists help diminish hepatic glucose production, further contributing to lower blood glucose levels.
Slowed Gastric Emptying: GLP-1 slows gastric emptying, which prolongs the feeling of fullness after eating and limits postprandial glucose excursions.
Appetite Suppression: GLP-1 receptor agonists have a central effect on appetite regulation, leading to decreased calorie intake and aiding weight loss, which is particularly beneficial for obese patients.
Weight Loss: The combined effects on appetite and calorie intake along with the enhanced glucose control contribute to significant weight loss in patients with obesity and T2D, which in turn can improve insulin sensitivity.
CT-996 as a Novel GLP-1 Receptor Agonist
CT-996 has been designed to have biased signaling on the GLP-1 receptor. This design is intended to have several potential advantages over traditional GLP-1 receptor agonists:
Signaling Bias: By preferentially activating the cAMP pathway with minimal to no recruitment of ß-arrestin, CT-996 could lead to prolonged pharmacological activity. Since ß-arrestin recruitment is associated with receptor desensitization and internalization, its minimal recruitment by CT-996 is hypothesized to reduce these processes, thereby promoting sustained therapeutic effects.
Potential for Improved Tolerability: The avoidance of ß-arrestin recruitment may also help improve the tolerability of the drug, since a common side effect associated with GLP-1 receptor agonists is gastrointestinal distress. Limiting ß-arrestin involvement could theoretically lead to fewer side effects.
Favorable Pharmacokinetics: With robust in vivo activity and good oral bioavailability observed in preclinical studies, CT-996 offers the convenience of once-daily oral dosing, which can greatly improve patient adherence to long-term therapy for chronic conditions such as obesity and T2D.
Better Therapeutic Window: The molecule's biased signaling and design aim to provide a more favorable therapeutic window, maximizing the drug's efficacy while minimizing adverse effects.
In spite of these theoretical advantages, the actual efficacy and safety profile of CT-996 would need to be conclusively proven through rigorous clinical trials. The initial Phase 1 PK clinical trial results are encouraging in establishing the drug's tolerability and dosing regimen, but further data from Phase 1b and subsequent trials will be necessary to determine its efficacy and full safety profile, particularly for the treatment of obesity and T2D.
The science behind the use of GLP-1 receptor agonists for the treatment of obesity and Type 2 Diabetes (T2D) is quite established. GLP-1-based therapies have been a pillar in the treatment landscape for T2D and more recently for obesity. Several GLP-1 receptor agonists, such as liraglutide, exenatide, dulaglutide, and semaglutide, have been approved for use and have shown efficacy in clinical trials. The processes by which GLP-1 receptor agonists exert their effects—enhanced insulin secretion, reduced glucagon secretion, slower gastric emptying, and appetite suppression—are well-characterized and supported by a robust body of preclinical and clinical evidence.
However, some aspects of GLP-1 receptor agonist science remain subject to ongoing investigation and debate:
Signaling Bias: The concept of biased agonism—that a compound can selectively activate one signaling pathway over another—is a relatively recent development in pharmacology. The hypothesis is that by avoiding ß-arrestin recruitment, a GLP-1 agonist like CT-996 could lead to less receptor desensitization, decreased internalization, and improved long-term efficacy and tolerability. While these benefits are supported by preclinical evidence, the clinical significance of biased agonism and its translation into meaningful therapeutic effects are still being validated.
Oral Bioavailability: Most approved GLP-1 receptor agonists are injectable. The development of an oral formulation, such as CT-996, addresses patient convenience and compliance but also presents challenges in terms of bioavailability and the preservation of drug stability and activity through the gastrointestinal tract. The development of an effective oral GLP-1 receptor agonist is groundbreaking and less established compared to the injectable forms.
Long-Term Safety Profile: While the short-term efficacy and safety of GLP-1 receptor agonists are well-documented, long-term data are still accumulating, particularly in relation to cardiovascular outcomes and potential rare adverse effects. Ongoing studies continue to build the evidence base around the long-term use of these medications.
Specific Efficacy of New Drugs: Although the class effects of GLP-1 receptor agonists are well-established, each new molecule warrants individual investigation to confirm its therapeutic profile. For example, CT-996's specific pharmacodynamic effects, efficacy for weight loss and glycemic control, and side effect profile need to be proven in large-scale clinical trials.
Overall, the level of evidence supporting the processes by which GLP-1 receptor agonists affect glucose metabolism and body weight is high. However, the evidence supporting the advantages of biased signaling and the successful development of oral formulations of GLP-1 receptor agonists, such as CT-996, is still evolving. While early clinical trial data for CT-996 may be promising, comprehensive Phase 3 trials and post-marketing surveillance are essential to firmly establish its efficacy, safety, and place in therapy for obesity and T2D.
Clinical trial overview
Summary of Study Design for CT-996:
The study is a Phase 1 clinical trial, multi-centered and randomized where the goal is to assess the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of CT-996 in overweight or obese individuals as well as patients with Type 2 Diabetes Mellitus (T2DM). The study has a double-blind, placebo-controlled, dose escalation design and includes both single and multiple-ascending doses for overweight or obese participants and multiple doses for T2DM patients. The trial involves the administration of a drug or placebo to a cohort of approximately 118 participants, with the projected start and end dates in May 2023 and November 2024, respectively.
Participants will be assigned to either the experimental group taking the CT-996 drug or the placebo comparator group. Treatment-emergent adverse events will be monitored to assess safety and tolerability. The pharmacokinetics will be analyzed by determining the maximum observed drug concentration (Cmax) and studying the effects of a high-fat meal on the plasma concentration of CT-996.
Critiques of the Study Design:
Phase 1 Trials: These trials primarily focus on safety and tolerability, so while it will provide essential data on CT-996 in terms of adverse events and initial pharmacokinetic profiles, it will not assess the efficacy of CT-996 in treating obesity or T2DM.
Population Sample: The study includes both overweight or obese participants and those with T2DM. The variance in the population may provide a broad perspective but could also introduce variability in the results due to different metabolic states, which could affect drug metabolism and response.
Masking: The triple-blinding is a strength of the study, as it minimizes bias. However, maintaining the blind, especially if there are distinctive side effects associated with the active drug, can be challenging.
Dose Escalation: A dose-escalation study helps establish the maximum tolerated dose, but it can be time-consuming and may require adaptive trial designs to efficiently reach optimal dosing levels.
Operational and Technical Challenges:
Recruitment: Enrollment of a specific patient population, particularly one that is heterogeneous (obese individuals and T2DM patients), may pose recruitment challenges.
Compliance: Ensuring participant compliance with the dosing regimen and study visits can be challenging, particularly in a study involving multiple sites.
Data Management: A multi-center trial requires consistent data management across different locations to ensure the quality and integrity of the collected data.
High-fat Meal Study: The high-fat meal study aspect will require standardizing the meal and controlling the timing of the meal and dosing across participants, which could be logistically complex.
Safety Monitoring: The careful monitoring of adverse events across diverse populations and adjusting trial parameters accordingly can be complicated, especially when unexpected safety concerns arise.
Overall, this first-in-human study for CT-996 aims to establish a safety profile and initial pharmacokinetic data, both crucial for the development of the drug. Ensuring rigorous methodology and overcoming operational challenges will be critical for meeting the study's objectives.
The potential of this study to provide proof-of-concept (PoC) for the use of CT-996 in the treatment of obesity at Phase 1 is limited, given that Phase 1 studies are primarily designed to assess the safety and tolerability of a drug, rather than its efficacy in treating a particular condition. The primary end points of this study, which involve monitoring the incidence of treatment-emergent adverse events, are suitable for the objectives of a Phase 1 trial. However, they do not directly address the effectiveness of CT-996 in reducing body weight or improving metabolic parameters associated with obesity.
Secondary outcomes such as pharmacokinetic measurements (e.g., Cmax) and the assessment of CT-996's interaction with a high-fat meal are appropriate for understanding the drug’s absorption, distribution, metabolism, and excretion (ADME) profile. While these can indirectly inform potential efficacy through drug exposure data, they still do not constitute direct efficacy endpoints, which are more commonly evaluated in later phases (Phase 2/Phase 3).
For a proof-of-concept related to efficacy, future studies would need to include primary endpoints that directly measure changes in body weight, body composition, or relevant metabolic markers such as blood glucose, insulin levels, or HbA1c for T2DM patients.
The inclusion/exclusion criteria listed are typical for early trials, aiming to select a population that could give a clearer understanding of the drug's safety profile without the confounding factors of comorbidities or polypharmacy. However, this also means that the results may not be fully generalizable to all populations with obesity, especially those with comorbid conditions, which are common in people with obesity.
The study design seems adequate for Phase 1 objectives related to safety, but additional criteria and primary endpoints are necessary for later phases aimed at providing PoC for efficacy. Designing subsequent trials with endpoints that reflect treatment benefits in obesity will be important for establishing the therapeutic potential of CT-996.
Many of the newer agents in T2D, particularly SGLT2 inhibitors and GLP-1 receptor agonists, are being highlighted for their cardiovascular benefits, which is an important consideration given the risk of heart disease associated with T2D. The development of oral formulations of drugs typically administered via injection (like semaglutide as Rybelsus) marks an important advance in terms of improving the ease of administration for patients.
CT-996's role within the T2D treatment will depend on the results of clinical studies. However, here is some general guidance on factors that would determine the potential place of a new antidiabetic medication like CT-996 in the therapeutic landscape, based on common considerations:
Efficacy: CT-996 would need to demonstrate substantial efficacy in reducing blood glucose levels, indicated by parameters such as HbA1c reductions. Comparable or superior efficacy to current therapy options would be necessary for it to become a part of the standard care. For reference, in the Rybelsus PIONEER Phase 3 trial for T2D, Rybelsus achieved A1C reductions in the range of 1.0 to 1.1%.
Safety and Tolerability: A favorable safety profile with minimal side effects is crucial for chronic diseases like T2D where compliance is often a challenge. CT-996 should have fewer or less severe side effects than currently available treatments to enhance tolerability.
Weight Management: Given the association between T2D and obesity, if CT-996 aids in weight loss or at least does not contribute to weight gain, it could be more attractive, especially for patients struggling with both conditions. In Phase 3 studies, GLP-1 agonists demonstrated significant weight loss. Semaglutide showed an average weight loss of about 33.7 pounds (15.3 kg). Liraglutide led to weight loss of about 10.5 to 15.8 pounds (4.8 to 7.2 kg). Dulaglutide's weight loss data varies, but it's generally considered effective for moderate weight loss. Tirzepatide, a newer agent, has shown promising results in weight reduction, but specific numbers would depend on the dosage and study design.
Cardiovascular Outcomes: Treatments that offer cardiovascular benefits are increasingly preferred in T2D management due to the high risk of cardiovascular disease in this population. Positive effects on cardiovascular risk factors or outcomes would position CT-996 favorably. Semaglutide, liraglutide, and dulaglutide have demonstrated a reduced risk of major adverse cardiovascular events including heart attack, stroke and cardiovascular death.
Mechanism of Action: If CT-996 has a novel mechanism of action that is different from existing therapies, it might be used in patients who are not well-controlled on current medications or could be added to existing regimens.
Administration: Oral medications are often easier for patients to take regularly compared to injectables. As an oral medication, CT-996 could enhance adherence and patient preference.
Cost: Affordability and insurance coverage often impact a drug's adoption. If CT-996 is competitively priced, it might be more accessible to a broader patient population.
Effects on Comorbidities: If CT-996 also addresses other aspects of T2D, such as high blood pressure, dyslipidemia, or reduces the risk of other complications like nephropathy or neuropathy, it may become a preferred option.
Diabetes Progression: Drugs that not only control symptoms but also slow the progression of T2D or mitigate the decline in pancreatic beta-cell function could be particularly valuable.
Integration with Personalized Medicine: A medication that can be targeted to specific populations based on genetic, metabolic, or phenotypic characteristics might fit well into a personalized approach to diabetes care.
Provided CT-996 can meet these considerations, it would potentially find its niche within the current standards of diabetic care, either as a first-line option, an alternative for patients who cannot tolerate other treatments, or as an add-on therapy for individuals needing additional glycemic control. This would be contingent on the outcome of clinical trials and approval by regulatory bodies. Once in the market, post-market studies could further elucidate its position relative to existing therapies.
CT-PYY
Scientific thesis
The therapeutic rationale for developing a long-acting PYY analog for the treatment of Prader-Willi Syndrome (PWS) is grounded in the role of PYY as a gut-derived hormone that is involved in the regulation of appetite and satiety. Here is a more detailed explanation:
PYY and Appetite Regulation:PYY, short for Peptide YY, is naturally produced in the gastrointestinal tract in response to food intake. PYY sends signals to the brain, particularly to hypothalamic areas involved in controlling appetite, promoting a sensation of satiety and fullness which helps reduce food intake. It mainly does this through its agonist action on the Y2 receptor, one of the several receptors (Y1, Y2, Y4, and Y5) it can bind to.
PYY Deficits in Obesity:Research has indicated that individuals with obesity might have impaired PYY signaling, either through decreased secretion after meals or through alterations in the sensitivity of the PYY receptors. The result is a less effective satiety signal, which can contribute to greater food intake and weight gain.
Characteristics of Prader-Willi Syndrome:Prader-Willi syndrome is a genetic disorder marked by a number of metabolic challenges, one of the most striking being an almost constant sense of hunger (hyperphagia) and deficits in satiety signaling, which leads to chronic overeating (obesity). The insatiable hunger and compromised metabolism are major health concerns for individuals with PWS, making the development of an effective appetite-suppressing therapy crucial.
Therapeutic Rationale for PYY Analog in PWS:By creating a long-acting analog of PYY that can be administered subcutaneously, the intention is to provide a persistent stimulation of the PYY receptors, particularly the Y2 receptor, which should enhance satiety signals and help patients feel full more effectively after eating. With its selective agonistic action, the PYY analog could better manage the hyperphagia associated with PWS and, consequently, address one of the core issues in this syndrome: the compulsive overeating that leads to obesity and related health problems.
Additional Combination Approaches:The potential of combining PYY analogs with other incretins like GLP-1 (Glucagon-like peptide-1) or GIP (Gastric inhibitory polypeptide) is of interest because these hormones also play significant roles in appetite regulation and glucose metabolism. The additive or synergistic effects of such a combination could offer a more comprehensive approach to treating not only the appetite dysregulation in PWS but also other metabolic abnormalities associated with this condition and obesity in general.
Given that this is an investigational treatment, clinical trials are necessary to evaluate the efficacy and safety of the long-acting PYY analog, as indicated by the forthcoming Phase 1 SAD/MAD data expected in 2025. If successful, this approach carries the potential for a significant impact on the quality of life and health outcomes for individuals living with Prader-Willi syndrome and potentially other metabolic diseases.
The science underlying the therapeutic rationale for PYY analogs in treating Prader-Willi Syndrome (PWS) is based on well-documented physiological processes and has been studied extensively, although it is fair to say that the field is continually evolving. Below are the aspects of this science with regards to their establishment and areas of uncertainty or debate:
PYY and Appetite Regulation:The role of PYY in appetite regulation is a well-established finding. Numerous studies have demonstrated that PYY can reduce food intake in both animals and humans. The mechanism by which PYY influences satiety, predominantly through the Y2 receptor, is generally accepted, although the intricacies of its action in different tissues and conditions are areas of ongoing research.
PYY and Obesity:There is solid evidence to support that individuals with obesity may have lower levels of PYY or altered responses to this hormone. This has led to a hypothesis that PYY dysfunction may contribute to the pathophysiology of obesity. However, the cause-and-effect relationship between PYY levels and obesity is complex and the topic of continuing research.
Prader-Willi Syndrome and Hyperphagia:The relationship between Prader-Willi syndrome and its associated insatiable hunger is clearly established in the medical literature. PWS is a well-documented genetic disorder with a characteristic phenotype that includes hyperphagia, leading to obesity if not managed. The physiological underpinnings of this aspect of PWS are not fully understood, involving not just the gut hormones but also central neurological pathways.
Therapeutic Use of PYY Analog in PWS:While the science supports the potential for PYY analogues to suppress appetite, translating this into a therapeutic strategy for PWS is still in development. The use of PYY analogs or their effects specifically in the context of PWS has not been conclusively demonstrated in clinical trials, as these are still ongoing or have yet to take place. Therefore, while preclinical studies may be promising, the efficacy and safety of PYY analog treatment for PWS patients remain to be firmly established through clinical trials.
Combinations with Other Incretins:Combining PYY with other incretin hormones like GLP-1 or GIP has a rationale based on the synergistic effects observed in preclinical models. However, the intricacies of such combinations and their dosages, efficacy, safety profiles, and long-term impacts are complex and not yet fully established in clinical settings.
The Overall Level of Evidence: Preclinical evidence supporting PYY's role in appetite suppression is strong. Observational studies linking PYY dysfunction to obesity are substantial but not conclusive regarding the direction of causation. Clinical evidence for PYY analog treatment in PWS is emerging but is yet to reach maturity with ongoing trials. Evidence for combination therapies with other incretins is based on early-phase research and theoretical synergism.
In summary, while the basic science regarding PYY and appetite regulation is well-found, there remains a significant amount of uncertainty when it comes to using PYY analogs as a treatment for human conditions, particularly for complex disorders such as Prader-Willi Syndrome. The ongoing and future clinical trials are crucial to translate this basic understanding into practical and safe treatments.
Prader-Willi Syndrome (PWS) is a genetic disorder characterized by a number of endocrine, neurological, and behavioral abnormalities, with hyperphagia and obesity being major clinical concerns. The research on PYY's direct role in PWS specifically is relatively limited, but several studies have looked at PYY levels in individuals with PWS and their correlation with hyperphagia and obesity.
Here are some findings from the literature that help to support the connection between PYY and PWS:
Studies have observed that individuals with PWS may have abnormal levels of PYY. For instance, a study titled "Plasma ghrelin and PYY levels are inversely correlated with body weight and fat mass in children with Prader-Willi syndrome" published in "International Journal of Obesity" in 2005, found lower postprandial PYY levels in children with PWS compared to obese and lean controls. This suggests an abnormality in the satiety signaling that could contribute to the hyperphagia seen in PWS.
A study published in "The Journal of Clinical Endocrinology & Metabolism" in 2004 titled "Prader-Willi Syndrome Is Characterized by Elevated Fasting Plasma Peptide YY Concentrations" showed that fasted plasma PYY levels are elevated in individuals with PWS when compared to control groups matched by body mass index, body composition, and sex. This appears to suggest that PWS may be associated with abnormal regulation of PYY, possibly contributing to their unrelenting hunger.
In contrast, another study titled "Chewing gum for 15 minutes suppresses hunger but not the desire to eat and gut peptide responses in women" published in the journal "Appetite" in 2018 suggested that individuals with PWS have a normal postprandial PYY response, which adds complexity to the overall understanding of PYY's involvement in PWS.
PWS presents a significant challenge when it comes to understanding the endocrine and metabolic disturbances because of its multifaceted nature. The current literature suggests there might be alterations in the levels of various hormones involved in appetite regulation, including PYY, but there is no single consistent pattern across all studies, and the precise mechanisms remain unclear.
Given these mixed findings and the complexity of PWS, while PYY is clearly implicated in the overall physiology of appetite and could be a therapeutic target in PWS, its specific role in this syndrome is not definitively established. More research, especially in the form of well-designed clinical trials, is necessary to understand how PYY analogs might be used effectively to manage symptoms of PWS, such as hyperphagia and obesity.
Here is a description of the strengths and weaknesses of this evidence base:
Strengths:
Physiological Mechanism: The strength of the evidence lies in the well-characterized physiological mechanism of action of PYY. PYY's role in reducing appetite is supported by numerous studies that demonstrate its ability to increase satiety and decrease food intake.
Study Consistency: There is consistent evidence from third-party studies in both humans and animals that show the appetite-reducing effects of PYY, which supports the rationality behind developing PYY analogs as a treatment to curb hyperphagia.
Observational Data: Research suggesting that individuals with obesity may have PYY abnormalities adds biological plausibility to the hypothesis that restoring or enhancing PYY signaling could be beneficial in conditions associated with overeating, including PWS.
Biological Pathway Involvement: The identification of PYY receptors and their role in the gastrointestinal system and brain indicates a specific pathway through which PYY analogs could exert a therapeutic effect.
Preclinical Studies: Animal studies and preclinical research demonstrating the effectiveness of PYY analogs provide foundational evidence that supports potential therapeutic effects in humans.
Weaknesses:
Translation to Clinical Practice: A major weakness is the gap in translating findings from basic scientific research and preclinical studies to effective clinical treatments. The path from understanding a biological mechanism to developing a safe and efficacious therapy is filled with challenges and potential unforeseen hurdles.
Specificity for PWS: The evidence base for the effects of PYY specifically in individuals with PWS is much more limited. There is a need for more targeted research to understand the nuances of how PYY functions in the unique metabolic and neuroendocrine environment of PWS.
Complexity of PWS: PWS is a complex genetic condition with a wide spectrum of symptoms, and managing hyperphagia alone may not address all the health issues faced by individuals with PWS. The interplay of multiple hormones and signaling pathways involved in PWS suggests a complex network that is not yet fully understood.
Variability in Response: Individual variability in response to PYY or its analogs is not well characterized. Factors such as genetic differences, degree of obesity, and other individual metabolic factors could affect treatment outcomes.
Long-term Safety and Efficacy: Long-term safety and the efficacy of PYY analogs have not been established. Potential side effects and the sustainability of therapeutic effects over time remain uncertain.
Evidence from Human Trials: There is a lack of substantial clinical trial data in human subjects, especially in PWS patients, which is necessary to confirm the efficacy and safety of PYY analog therapy in the intended population.
Overall, while there is a robust theoretical and biochemical framework supporting the exploration of PYY analogs as a therapy for conditions like PWS, there is a need for more direct evidence from clinical trials to substantiate the use of PYY analogs as an effective treatment. As such treatments progress through phases of clinical research, the evidence base will hopefully expand to provide more definitive answers regarding the therapeutic utility of PYY analogs.
Market overview
Prader Willi syndrome
Prader-Willi syndrome (PWS) is a rare and complex genetic disorder that affects multiple systems of the body. Characterized by symptoms such as hypotonia (poor muscle tone), hyperphagia (an unrelenting drive to eat frequently leading to obesity), intellectual disability, and endocrine disorders, including growth hormone deficiency, there is a significant unmet medical need for effective treatments for this condition.
There is no cure for PWS, and the standard of care predominantly involves symptom management through a multidisciplinary approach. Growth hormone therapy is commonly prescribed to address growth deficiency and can have a positive impact on body composition and physical strength. Strict dietary management and supervision, as well as behavioral therapies, are also key components in managing the syndrome.
The market opportunity in Prader-Willi syndrome may be seen in several areas:
Therapeutic Avenues: Since PWS is a spectrum disorder with a variety of symptoms, therapeutic strategies that target specific aspects of the syndrome such as hyperphagia, obesity, growth deficiency, or mental health issues could be valuable. Drugs that offer better control of these symptoms could gain a strong market position.
Orphan Drug Designation: PWS is recognized as an orphan disease due to its rarity, a classification that often comes with incentives for pharmaceutical companies, such as market exclusivity, tax credits for clinical research, and waiver of marketing application fees, to develop treatments.
Pipeline Drugs: Several pipeline drugs targeting PWS are under investigation. Success of these drugs in clinical trials could lead to substantial market opportunities due to the limited competition.
High Unmet Need: The unmet needs in PWS are significant. There is a particular interest in treatments that can effectively control appetite and those that can improve cognitive function. Companies that develop novel drugs with proven efficacy and safety in these areas could capture a significant market share.
Few specific treatments have been approved solely for the treatment of PWS. However, drugs that are approved for other indications, like growth hormone for growth deficiencies, are often used off-label for patients with PWS. Any drug that is specifically designed for PWS will have to navigate clinical trials that prove superiority or at least non-inferiority to the current off-label treatments, while also demonstrating a well-tolerated safety profile.
In terms of the market size, the rarity of PWS implies a smaller patient population compared to common diseases. However, orphan drugs can still be commercially successful due to their ability to command higher prices and receive regulatory incentives. Moreover, breakthrough therapies for rare diseases can receive significant media attention, which can drive awareness and diagnosis rates, potentially enlarging the treatable market.
Companies operating in the PWS market need to be keenly aware of the dynamics of rare disease drug development and commercialization, including regulatory pathways, pricing and reimbursement challenges, and the importance of patient advocacy groups. Collaborations with these groups can improve trial design, recruitment, and provide support throughout the drug development process.
Comprehensive market analysis would require up-to-date data on incidence and prevalence of PWS, current treatment costs, pricing strategies for orphan drugs, competitor analysis, and in-depth understanding of regional health care systems, reimbursement policies, and patient access programs.
There are several promising treatments in development for Prader-Willi syndrome that are targeting various aspects of the disorder:
Levo Therapeutics' LV-101 (intranasal carbetocin): Carbetocin is an analog of the naturally occurring hormone oxytocin and is being studied for its potential to reduce hyperphagia and improve behavioral symptoms in PWS. Levo Therapeutics has been actively involved in clinical trials to evaluate its efficacy and safety. Phase 3 studies have been conducted, and results from these could determine its potential for FDA approval.
Soleno Therapeutics' DCCR (diazoxide choline controlled release): DCCR is an orally administered, once-daily tablet that is designed to control hyperphagia and obesity in patients with Prader-Willi syndrome. Diazoxide choline is an ATP-sensitive potassium channel agonist that may have an indirect effect on reducing appetite. The company has faced regulatory hurdles but has continued development in light of the significant unmet needs of the PWS population.
Millendo Therapeutics' Livoletide (MZ-610): Livoletide is an unacylated ghrelin analog that might regulate appetite and has been investigated for reducing excessive eating and improving behavior in individuals with PWS. Previous studies have suggested benefits, although a Phase 2b/3 trial did not meet its primary efficacy endpoint.
Genetic Therapies: CRISPR and other gene-editing technologies are being considered for their long-term potential in treating genetic disorders like PWS. By targeting the root genetic causes, these therapies might one day provide a cure. However, this research is still in the early stages, and clinical use—if proven safe and effective—is likely several years away.
Oxytocin Analogs: Since PWS patients may have deficiencies in oxytocin or its receptors, treatments using oxytocin analogs are being studied. Intranasal oxytocin has been investigated as a potential treatment for improving social behaviors and reducing food intake, with mixed results.
Pitolisant: Originally developed for narcolepsy, pitolisant has also been considered for PWS given its mechanism of action as a selective histamine H3 receptor antagonist/inverse agonist, which may affect wakefulness and appetite control.
It's important to note that while these treatments are promising, they each have hurdles to overcome in terms of demonstrating efficacy, safety, and tolerability in a challenging and varied patient population like that of PWS. Clinical trials are critical to determine whether these potential treatments can make a meaningful impact on the lives of patients with PWS, and gaining regulatory approval will require robust data to support their benefits.
Additionally, any drug development for PWS will need to address the complexities of the syndrome, which includes not only hyperphagia and obesity but also developmental issues, psychiatric conditions, and endocrine disorders. Therefore, multi-faceted therapeutic approaches and personalized medicine strategies might be necessary for effectively managing the syndrome.
Partnering with patient advocacy groups and involving patients and caregivers in the design of clinical trials can help ensure that the outcomes measured are meaningful to those affected by PWS. As treatments progress through the pipeline, researchers, clinicians, and pharmaceutical companies will continue to refine their strategies and hopefully bring new and effective therapies to market for this complex condition.
There are no drugs specifically approved to treat the core genetic cause or the full spectrum of symptoms associated with Prader-Willi syndrome (PWS). Treatment is primarily focused on managing specific symptoms and associated conditions. Here are some notable approaches to treatment:
Growth Hormone Therapy: One of the most significant treatments for PWS is the use of growth hormone. Growth hormone therapy was approved by the FDA for use in children with PWS to improve growth, body composition, and physical strength. Drugs like Genotropin, Norditropin, and others are examples of recombinant human growth hormones that are used in the treatment of PWS, though they are not branded specifically for PWS.
Oxytocin and Analogues: While not necessarily branded drugs for PWS and not having received formal FDA approval for treating PWS, oxytocin has been studied for its effects on social behavior and feeding patterns in PWS patients. Researchers have also been exploring carbetocin, an oxytocin analogue, for potential effects on hyperphagia and social cognition.
None of these address the full range of symptoms experienced by individuals with PWS nor do they treat the underlying genetic cause of the syndrome. This underlines the significant unmet need for treatments within the PWS community.
PYY, or Peptide YY, is a naturally occurring hormone in the body that is released by the small intestine in response to food intake. It has been shown to decrease appetite and inhibit gastric motility. It works as part of an "ileal brake" mechanism to signal satiety and fullness to the brain, thus reducing food intake.
A drug like CT-PYY could potentially fit into the standard of care for PWS by addressing one of the most challenging symptoms of PWS: hyperphagia. Hyperphagia is the excessive sense of hunger leading to overeating, which can cause severe obesity and related health issues in PWS patients. By contributing to the feeling of satiety, CT-PYY might help control this symptom, potentially reducing the risk of obesity and its related complications.
Where CT-PYY would fit into the standard care might depend on several factors:
Efficacy: The drug would need to demonstrate through clinical trials that it substantially reduces hyperphagia without significant adverse effects. The effect on weight management and overall quality of life for PWS patients would also be important factors.
Safety: Since patients with PWS often have complex medical profiles, including hypotonia, sleep abnormalities, and a higher risk for gastrointestinal issues, it's crucial that CT-PYY is safe for long-term use and does not introduce further complications.
Approval and Accessibility: CT-PYY would need to gain regulatory approval and be accessible to patients. This would involve setting a price that considers the orphan drug status typically granted to PWS medications but is also acceptable to healthcare payers.
Competition: The landscape of PWS treatments is evolving. Depending on the timeline for development and approval for CT-PYY, other treatments may establish themselves in the market, which could affect the adoption of CT-PYY.
Combination with Other Treatments: CT-PYY might be used in combination with other treatments like growth hormone therapy or behavioral interventions. Clinical trials would need to evaluate the safety and efficacy of such combination approaches.
Endorsement by Medical Community and Advocacy Groups: Acceptance by healthcare professionals specializing in PWS and support from patient advocacy groups would enhance the potential for CT-PYY to be integrated into standard care. These entities help guide practice through their recommendations and influence payer coverage decisions.
Monitoring and Support: Given the complexity of PWS and the potential need for lifelong treatment, a medication like CT-PYY would likely require a support program to monitor patients, manage dosing, and provide education on managing PWS symptoms.
If CT-PYY successfully addresses these factors, it could potentially become an integral part of the multi-faceted approach to managing PWS, specifically targeting hyperphagia and its ensuing complications. However, without the specific text from the company's website or access to detailed data on CT-PYY's development, this analysis remains speculative and theoretical. Further information from clinical trials and continued research would be necessary to make a more precise determination of how CT-PYY might become part of the PWS treatment paradigm.