CG Oncology IPO investment analysis
January 8, 2024
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Overview
CG Oncology is a late-stage biopharmaceutical company developing cretostimogene, an investigational therapeutic for bladder cancer. The company is currently advancing the product towards commercialization, focusing on high-risk Non-Muscle Invasive Bladder Cancer (NMIBC) patients unresponsive to standard Bacillus Calmette-Guérin (BCG) therapy.
Cretostimogene is undergoing a Phase 3 clinical trial (BOND-003) to assess its safety and efficacy as a monotherapy. Interim results have shown promising rates of complete response (CR) with 75.7% of patients achieving CR at some point during the trial. A substantial number of responders maintained their response for at least six months without significant treatment-related adverse events.
Concurrently, CG Oncology is conducting the CORE-001 Phase 2 clinical trial combining cretostimogene with pembrolizumab, an FDA-approved checkpoint inhibitor. Preliminary results also show high percentages of CR over various time points with few serious adverse events.
The company is exploring cretostimogene's use in additional bladder cancer indications, such as intermediate-risk NMIBC post-surgery and potentially for Muscle Invasive Bladder Cancer (MIBC).
Cretostimogene's clinical advantage includes intravesical administration compatible with existing BCG therapy practices and its therapeutic potential, shown to induce notable disease control and safety outcomes over six months in the Phase 3 BOND-003 trial. Phase 2 CORE-001 data indicates its effective combination with pembrolizumab, suggesting versatility in combination regimes.
Scientifically, cretostimogene leverages a mechanism of action that may stimulate a localized immune response within the bladder, engaging the patient's immune system to target and eliminate cancer cells. This contrasts with the systemic effects of traditional chemotherapeutics and could reduce overall treatment burden and systemic toxicity.
Given successful interim trial results, FDA fast track designation, and the absence of severe treatment-related adverse effects, cretostimogene shows promise as a potential primary therapy that could revolutionize NMIBC management and extend its therapeutic application to broader bladder cancer indications. The company projects topline data from BOND-003 by the end of 2024, with positive results possibly leading to a Biologics License Application (BLA) submission to the FDA.
Pipeline overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| Cretostimogene | Viral therapy | Retinoblastoma gene pathway-defective cells Oncolytic immunotherapy | BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer | |||||||
| Cretostimogene | Viral therapy | Retinoblastoma gene pathway-defective cells Oncolytic immunotherapy | BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer in combination with pembrolizumab | |||||||
| Cretostimogene | Viral therapy | Retinoblastoma gene pathway-defective cells Oncolytic immunotherapy | Intermediate-Risk Non-Muscle Invasive Bladder Cancer | |||||||
| Cretostimogene | Viral therapy | Retinoblastoma gene pathway-defective cells Oncolytic immunotherapy | BCG-exposed and BCG-naive Non-Muscle Invasive Bladder Cancer |
Highlights and risks
Strong clinical data from well-designed trials
Targeting indication with significant unmet need
Near-term FDA approval upon positive Phase 3 data
Potential near-term M&A candidate upon positive Phase 3 data or approval
Total number of patients with BCG-unresponsive high-risk NMIBC is comparatively small
Larger opportunity in BCG-naive NMIBC, but clinical data must be competitive vs. BCG to support penetration into market
Valuation
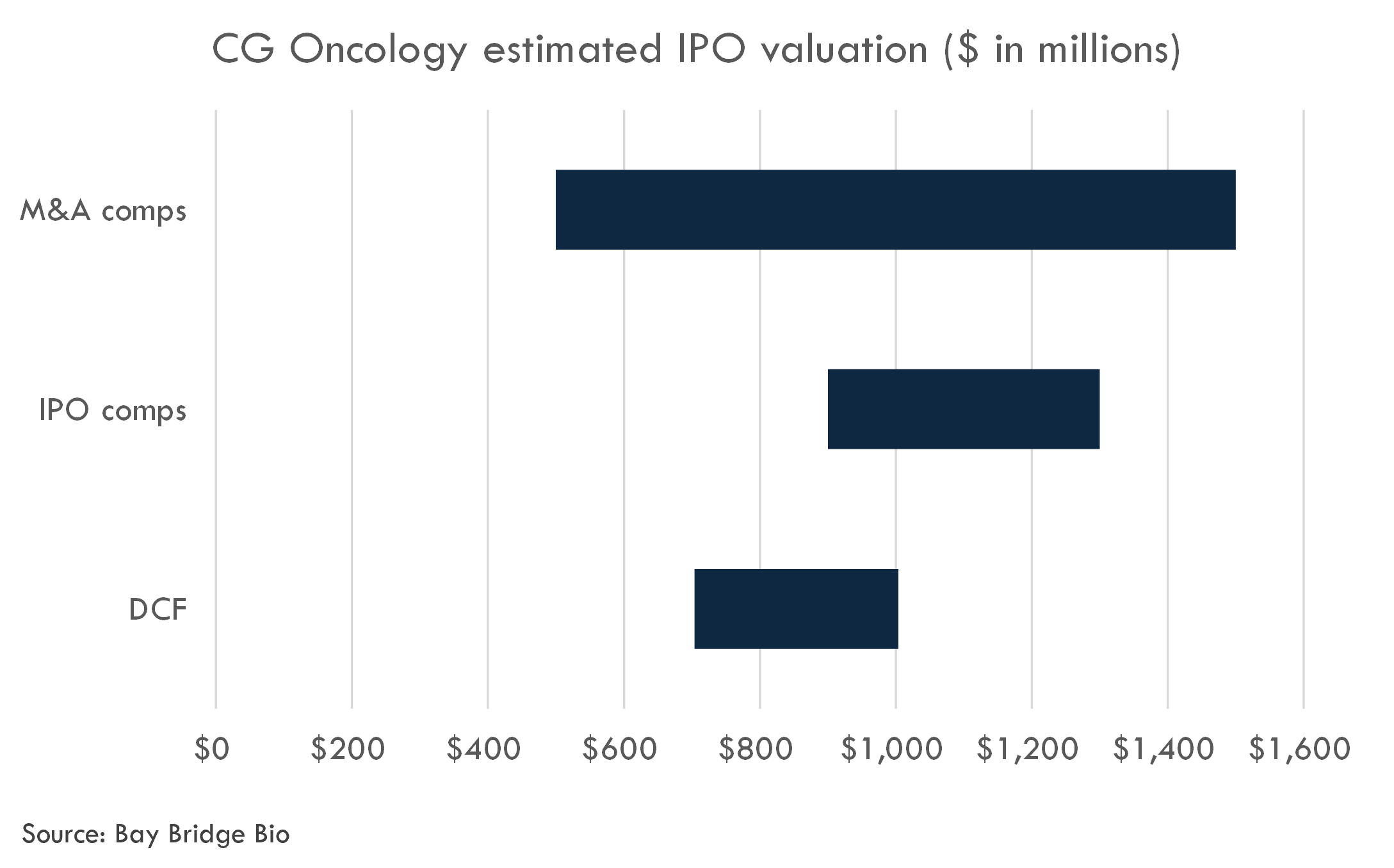
CG Oncology filed to go public in January 2024. The company's estimated post-money valuation of its latest round is $583 million.
We estimate an IPO valuation range of $900 million to $1,300 million. This is supported by M&A comps analysis as well as a Discounted Cash Flow analysis.
Based on comparable recent IPOs, the following investors could potentially be candidates for investing in CG Oncology's IPO:

Weekly analyses of biotech startups, generated by AI
Receive high quality, AI-generated analyses of biotech startups, public companies and scientific papers each week.
Cretostimogene
Scientific thesis
Oncolytic immunotherapy is a promising therapeutic approach for treating various cancers, including bladder cancer. In the context of Non-Muscle Invasive Bladder Cancer (NMIBC), especially in high-risk cases where treatment options are limited, the development of novel treatments like cretostimogene—engineered to selectively target and destroy cancer cells while stimulating an anti-tumor immune response—is significant. Here is a rationale for using oncolytic immunotherapy in different NMIBC settings:
BCG-Unresponsive High-Risk NMIBC: Bacillus Calmette-Guérin (BCG) is the standard intravesical immunotherapy for NMIBC, but not all patients respond to it, and some may have a recurrence of cancer after BCG therapy. These patients are considered BCG-unresponsive and are at a higher risk of the disease progressing to a more invasive state. Cretostimogene offers an alternative therapeutic approach for these patients by directly killing cancer cells and potentially alerting the immune system to the presence of cancer. This may limit the need for radical treatments like cystectomy, which have significant impacts on quality of life.
BCG-Unresponsive High-Risk NMIBC in Combination with Pembrolizumab: Pembrolizumab is a checkpoint inhibitor that blocks the interaction between PD-1 and its ligands, thereby enhancing T-cell responses against cancer cells. The combination of cretostimogene with pembrolizumab may synergize to enhance the immune-mediated destruction of tumor cells. The early clinical data showing high rates of complete response (CR) suggests that this combination could be a potent treatment regimen for high-risk NMIBC.
Intermediate-Risk NMIBC: Patients with intermediate-risk NMIBC have a lower risk of progression than those with high-risk NMIBC but would still benefit from more effective treatments than current standard of care. The rationale for using cretostimogene in this group is to potentially offer a more effective treatment that provides durable responses while still preserving the bladder.
BCG-Exposed and BCG-Naive NMIBC: Cretostimogene is also being explored in patients who have been exposed to BCG but have not reached a point of unresponsiveness, as well as in BCG-naïve patients. For BCG-exposed patients, cretostimogene may help overcome resistance to BCG and induce a stronger immune response. For BCG-naïve patients, cretostimogene may serve as an initial, potentially more effective treatment, or it could be offered to patients for whom BCG is not indicated for medical reasons.
Cretostimogene's mode of action involves two key modifications for tumor selectivity and potency: the first ensures the virus preferentially replicates in Rb-deficient cancer cells, and the second involves the expression of GM-CSF to stimulate a robust anti-tumor immune response. This dual mechanism of directly lysing tumor cells and stimulating the immune system positions cretostimogene as a highly promising candidate for NMIBC.
Administration of cretostimogene via intravesical delivery is minimally invasive and can be conveniently done in an office setting, which is favorable compared to more invasive or systemic therapies. The strong safety profile, as indicated by the lack of severe treatment-related adverse events (TRAEs), further supports its therapeutic potential.
Ultimately, the goal of oncolytic immunotherapy in NMIBC is to provide a bladder-preserving, effective treatment that reduces the chances of disease progression and avoids the morbidity associated with more aggressive interventions like cystectomy. The ongoing clinical trials for cretostimogene will provide critical data to determine its efficacy and safety in different NMIBC subpopulations and potentially fill a significant unmet need in bladder cancer therapy.The science behind oncolytic immunotherapy and its mechanisms of action has been progressing and accumulating evidence over the past few decades, but it's still a relatively new field compared to traditional cancer treatments like chemotherapy, radiation, and surgery.
Key Established Points:
Oncolytic Viruses (OVs): The concept of oncolytic viruses selectively targeting and killing cancer cells is well-established. The FDA approval of talimogene laherparepvec (T-VEC) for melanoma in 2015 provided a significant validation for oncolytic virotherapy.
Immunogenic Cell Death: The idea that the death of cancer cells can release tumor antigens and induce an adaptive immune response against the tumor is well-supported in scientific literature and is a central rationale for combining oncolytic viruses with immunotherapies.
GM-CSF: The use of granulocyte-macrophage colony-stimulating factor (GM-CSF) to enhance immune responses is not new and has been applied in various therapeutic cancer vaccines and treatments.
Mechanisms of Pembrolizumab: The mechanism of action of checkpoint inhibitors like pembrolizumab is established, and these drugs are approved for various cancers. They have been shown to reactivate the immune system against tumors.
Uncertainties and Areas of Debate:
Tumor Selectivity: While cretostimogene is engineered to target Rb pathway-defective cells, the specificity and efficiency of this targeting in humans are complex issues, and the potential for off-target effects or resistance mechanisms are areas of ongoing research.
Optimal Combination Therapy: The best combination strategies of OVs with other cancer treatments, including checkpoint inhibitors, are still being explored. Determining the timing, dosage, and sequencing of such therapies for maximal efficacy and safety remains an active area of research.
Durability of Response: Although early clinical trials show promising results in terms of response rates, the long-term durability of these responses and the ability to prevent cancer recurrence or progression over many years is still uncertain. More data are needed from longer follow-up periods.
Biomarkers: Identifying reliable biomarkers that predict which patients will respond to oncolytic immunotherapies and which will not is still an ongoing challenge.
Level of Evidence:
The evidence for cretostimogene's efficacy and safety in NMIBC is still emerging, primarily from Phase 2 and Phase 3 clinical trials. Such trials provide a higher level of evidence than preclinical or early-phase studies, but complete and long-term data are not yet available, as many trials are ongoing and data are still being collected and analyzed. Peer-reviewed publications, full clinical trial data, and multiple trials demonstrating similar outcomes would raise the level of evidence.
Regulatory agencies like the FDA consider data from well-designed clinical trials to be the gold standard in determining the safety and efficacy of new therapies. Until cretostimogene receives FDA approval based on sufficient evidence from these clinical trials, its use remains investigational.
Mechanism of action overview
The retinoblastoma (Rb) pathway plays a critical role in cell cycle regulation, and its dysfunction is implicated in the pathogenesis of various cancers, including bladder cancer. The Rb protein interacts with E2F transcription factors to control the progression from the G1 to the S phase of the cell cycle. When the Rb pathway is defective, unregulated cell division can occur, contributing to the development and progression of cancer.
In the context of NMIBC, the Rb gene pathway may be particularly relevant for a few reasons:
Rb Pathway in Bladder Cancer Prognosis and Treatment Response: Some studies have indicated that alterations in the Rb pathway could be associated with more aggressive bladder cancer and poor prognosis. For instance, the loss of Rb expression has been associated with higher stage and grade tumors, and these alterations might predict the response to therapies like chemotherapy.
BCG-Unresponsive High-Risk NMIBC: There is evidence suggesting that high-risk NMIBC may display alterations in the Rb pathway, and these alterations could be associated with the response to BCG treatment. Rb pathway alterations could potentially contribute to the development of BCG-unresponsive disease by enabling cancer cells to evade BCG-induced immunity or apoptosis.
Oncolytic Virus and Rb Pathway: Oncolytic viruses such as cretostimogene have been designed to selectively replicate in Rb pathway-defective cells, offering a targeted approach for tumor cell lysis. This specificity not only direct kills cancer cells but may also initiate a broader immune response by releasing tumor antigens upon cell lysis.
Combinational Therapy with Pembrolizumab: As oncolytic viruses may increase tumor antigen release and dendritic cell priming, they could work synergistically with checkpoint inhibitors like pembrolizumab, which aim to rejuvenate the T-cell-mediated anti-tumor immune response. Pembrolizumab has been approved across various types of cancer and has shown activity in bladder cancer, suggesting that pairing it with an oncolytic virus targeting Rb pathway alterations in bladder cancer could be a potent combination.
Relevance in BCG-Exposed and BCG-Naive NMIBC: The role of Rb pathway in BCG-exposed and naive NMIBC patients is yet to be fully elucidated. However, early detection of pathway alterations could help in stratifying patients and potentially offer a more targeted oncolytic virus treatment for those with defective pathways, regardless of previous BCG exposure.
Although the rationale and preliminary findings are promising, further in-depth research is warranted to establish a solid connection between Rb pathway defects and therapeutic response in both BCG-unresponsive and BCG-naive NMIBC patients. Direct literature links specifically correlating the Rb gene pathway-defective cells’ role in these specific contexts of bladder cancer are sparse and may be primarily sourced from ongoing clinical trial results like those reported for cretostimogene.
The evidence base supporting the therapeutic rationale for the use of oncolytic virotherapy, and in particular cretostimogene, in the treatment of Non-Muscle Invasive Bladder Cancer (NMIBC) comes primarily from preclinical studies and clinical trials. The strengths and weaknesses of this evidence can be outlined as follows:
Strengths:
Targeted Mechanism of Action: There is a strong biological plausibility underlying oncolytic virotherapy, with viruses engineered to selectively target cancer cells with certain genetic defects, such as Rb pathway deficiencies, which are common in urothelial carcinoma.
Clinical Trial Data: Results from Phase 2 and 3 clinical trials provide direct evidence of the effectiveness and safety of cretostimogene in NMIBC. The reported response rates and durations, along with favorable safety profiles, support the therapeutic potential of this approach.
Immune Stimulation: Oncolytic viruses like cretostimogene provide a dual mechanism of action by causing direct lysis of tumor cells and inducing an immunogenic cell death that can trigger systemic anti-tumor immune responses, leading to durable benefits.
Synergy with Immunotherapy: Early evidence from trials combining oncolytic virotherapy with immune checkpoint inhibitors (like pembrolizumab) suggests an enhanced therapeutic effect, leveraging the strengths of each approach.
Weaknesses:
Early Stage of Development: While clinical trial results are promising, the most advanced trials are still in Phase 3. Full approval of cretostimogene and broad acceptance of its use will require completion of these trials and possibly additional confirmatory studies.
Lack of Long-Term Data: Many clinical trials have short follow-up periods, which do not provide sufficient information about the long-term efficacy and safety of the treatment.
Limited Population: Clinical trials are conducted under strict inclusion/exclusion criteria, which may not represent the broader patient population that would receive the therapy after approval. It is unclear how cretostimogene will perform in the real-world setting.
Regulatory Hurdles: Full regulatory approval is pending, with most evidence coming from single-arm studies rather than randomized controlled trials, which are considered the gold standard by regulatory authorities.
Biomarker Identification: Identification of biomarkers to predict response to oncolytic virotherapy is still an area of ongoing research, and there is a lack of validated predictive biomarkers in NMIBC for this treatment modality.
Generalizability: Response rates to oncolytic viruses and immune checkpoint inhibitors can vary widely among individuals, potentially due to differences in tumor immunogenicity, viral infectivity, host immunity, and other factors not yet fully understood.
In summary, the evidence base for the therapeutic rationale of cretostimogene in NMIBC is developing with several indications of potential efficacy and a reasonable safety profile based on mid-stage clinical trial data. However, it is challenged by the limitations inherent in the study designs, the potential need for longer-term data, the applicability to a broader patient population, and the need for deeper understanding of the predictive biomarkers for responsiveness to treatment. Further research and completion of ongoing clinical trials will address these weaknesses and contribute to the establishment of a more comprehensive evidence base.
Clinical trial overview
BOND-002
BOND-002, titled "Safety and Efficacy of CG0070 Oncolytic Virus Regimen for High Grade NMIBC After BCG Failure (BOND2)", is a Phase II, multicenter, single-arm interventional trial. It was designed to evaluate the safety and efficacy of CG0070, an oncolytic virus expressing GM-CSF, in patients with high-grade Non-Muscle Invasive Bladder Cancer (NMIBC) who have failed BCG therapy and refused cystectomy.
Study design summary:
- Objective: To assess CG0070 in high-grade NMIBC patients post-BCG therapy failure.
- Intervention: CG0070 administered at a dose of 1e12 vp weekly for six weeks. Responding patients received maintenance therapy.
- Follow-Up: Patients monitored every 3 months up to 24 months.
- Primary Outcome: Durable Complete Response Proportion (DCR) for at least 18 months post-first intravesical intervention.
- Secondary Outcomes: Several measures, including Cystectomy Free Survival and Progression Free Survival, with an 18-month timeframe post-treatment.
Below are some critiques of the study design:
- Single-Arm, Open-Label Design: Without a control group, it's challenging to attribute outcomes solely to the intervention, particularly in terms of safety and efficacy. The open-label nature may introduce bias.
- Subjective Outcome Measures: Durable Complete Response as a primary outcome is somewhat subjective and may be influenced by variables other than the treatment efficacy.
- Lack of Data Collection: Several secondary outcomes, such as Cystectomy Free Survival and Complete Response Survival, were noted as "not available or not collected." This omission could limit the understanding of the treatment's comprehensive impact.
- Limited Generalizability: The study focuses on a specific subset of NMIBC patients who have failed BCG therapy and refused cystectomy, which may not represent the broader NMIBC patient population.
- Follow-Up Duration: While a 24-month follow-up is reasonable, longer-term data would be necessary to fully understand the durability of the treatment effects and long-term safety.
Inclusion criteria: The criteria focus on patients with high-grade NMIBC, a history of BCG failure, and those refusing cystectomy. This specificity is appropriate as it targets a patient population with significant unmet medical needs. The requirement for pathologically confirmed HG NMIBC and no evidence of muscle invasive disease is critical to ensure the study population is homogeneous and relevant. The stipulation of prior BCG therapy and the timeframe of disease recurrence post-treatment are important for defining BCG failure.
Exclusion criteria: Excluding patients with previous systemic chemotherapy or radiation for bladder cancer is appropriate, as these treatments could confound the effects of CG0070. Other exclusions, such as hypersensitivity to GM-CSF or infections, are standard to minimize safety risks. The exclusion of patients on immunosuppressive therapy is crucial, given the potential impact on the effectiveness of an oncolytic virus.
Results
-
Clinical Data Summary for Cretostimogene in BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC):
-
Response Rate:
- The clinical data indicated a 65% complete response (CR) rate among 46 patients with high-risk carcinoma in situ (CIS)-containing NMIBC following cretostimogene treatment.
- Subsequent re-dosing at three months resulted in an additional 40% of the patients who had not previously achieved CR reaching CR at the six-month mark.
-
Duration of Response (DOR):
- The durability of the treatment response was promising, with 44% of patients sustaining CR at six months and 28% maintaining CR at one year after treatment.
-
Safety and Tolerability:
- Cretostimogene appeared generally well-tolerated among 68 patients (65 per protocol and three additional patients for compassionate use or due to retrospective disqualification).
- The treatment-related adverse events (TRAEs) were mostly confined to Grade 1 and 2 in severity.
- There were only two Grade 3 TRAEs reported, both dysuria and hypotension, and both were resolved without any Grade 4 or 5 TRAEs recorded.
- Eight serious adverse events (SAEs) occurred, but none were related to cretostimogene.
-
Conclusion:
- The clinical data from the BOND-002 trial suggest that cretostimogene is a potentially effective and generally well-tolerated treatment option for patients with BCG-unresponsive high-risk NMIBC, especially for those who are not candidates for or have refused cystectomy.
- Both the response rates and the duration of response highlight cretostimogene's therapeutic potential, alongside a manageable safety profile.
-
Response Rate:
BOND-003
BOND-003 is a Phase 3 clinical trial evaluates the efficacy and safety of CG0070, an engineered oncolytic adenovirus, in patients with non-muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus-Calmette-Guerin (BCG) therapy. The study involves intravesical (IVE) administration of CG0070 along with a transduction-enhancing agent, n-dodecyl-B-D-maltoside (DDM).
Key aspects include:
- Population: Up to 110 patients with carcinoma in situ with or without concomitant high-grade Ta or T1 papillary disease.
- Treatment Protocol: CG0070 is administered weekly for six weeks, with further cycles dependent on the patient's response at Week 13. Maintenance therapy continues with treatments every 12 weeks (up to Week 49) and then every 24 weeks.
- Primary Outcome: Complete response rate in patients with carcinoma in situ (with or without high-grade Ta or T1 papillary disease) over 24 months.
- Secondary Outcomes: Median Duration of Response (DOR), Median Progression-Free Survival, Time to Tumor Progression (TTP), Incidence of adverse events, and Comparison of complete response in patients with persistent versus relapsed disease at baseline.
Below are some critiques of the study design:
- Open-Label, Single Arm: Without a control or comparative arm, it's challenging to differentiate the effects of CG0070 from natural disease progression or placebo effects. The lack of blinding can introduce bias in outcome reporting.
- Patient Population: The study is specific to NMIBC patients who are unresponsive to BCG, which is beneficial for targeted therapy but may limit the generalizability of results to broader NMIBC populations.
- Primary Outcome: While complete response rate is a relevant and clear endpoint, reliance on this alone may not fully capture the nuances of cancer progression, particularly in NMIBC.
- Treatment Schedule: The complexity and length of the treatment protocol may pose challenges in patient compliance and consistent administration.
Operational and technical challenges include:
- Recruitment and Retention: Enrolling a sufficient number of patients who meet the specific criteria of BCG unresponsiveness and maintaining their participation over a long treatment and follow-up period can be challenging.
- Treatment Administration: Ensuring consistent and accurate intravesical administration of CG0070 and DDM, particularly over an extended period, requires rigorous procedural adherence and could be resource-intensive.
- Response Assessment: Regular and accurate assessment of tumor response, particularly in distinguishing complete responses from partial responses or stable disease, requires meticulous and standardized evaluation, which can be operationally demanding.
- Safety Monitoring: Given that CG0070 is a novel treatment, close monitoring for adverse events is crucial. The open-label design could influence the reporting and interpretation of these events.
- Data Collection and Analysis: The complexity of the treatment schedule and the prolonged follow-up period may complicate data collection and analysis, particularly in ensuring consistency and managing missing data.
Results
-
Response Rates:
- Out of 66 evaluable patients, 50 (75.7%) achieved a complete response (CR) at any time following treatment with cretostimogene.
- At the three-month mark, 45 patients (68.2%) had achieved CR.
- By six months, 42 patients (63.6%) maintained CR.
-
Re-dosing Efficacy:
- Among the 13 patients who did not attain CR at three months and were re-dosed, four (30.8%) subsequently achieved CR at six months.
- One patient who did not achieve CR by six months but continued the treatment per the principal investigator's recommendation attained CR at nine months (although not included in the results as per protocol).
-
Durability of Response:
- Of the patients who achieved CR at any time, 42 out of 50 (84.0%) maintained the response for at least three months.
- For those evaluable for a six-month duration, 32 out of 43 (74.4%) maintained their CR for at least six months.
- Seven patients were not included in the six-month durability analysis as they had not reached the minimum duration for evaluation.
-
Safety and Tolerability:
- Cretostimogene showed a tolerable safety profile with most adverse events being Grade 1 or Grade 2.
- No Grade 3 or higher treatment-related adverse events (TRAEs) were reported.
- There were no instances of treatment discontinuation due to adverse events.
- No deaths have been reported.
- Only two patients (1.8%) experienced serious adverse events (SAEs), including Grade 2 noninfective cystitis and Grade 2 clot retention, both resolved.
-
Conclusion:
- Based on the interim results from the BOND-003 trial, cretostimogene demonstrates a high rate of effectiveness in inducing complete remission in patients with high-risk NMIBC who are unresponsive to BCG treatment.
- The treatment also shows a reasonable duration of response and has an acceptable safety and tolerability profile, with no high-grade TRAEs or treatment discontinuations reported to date.
CORE-001
CORE-001 is a Phase 2, single-arm, open-label clinical trial designed to evaluate the efficacy and safety of the combination of CG0070, an engineered oncolytic adenovirus, and pembrolizumab, an immune checkpoint inhibitor, in patients with high-risk non-muscle invasive bladder cancer (NMIBC) who have not responded to Bacillus Calmette-Guerin (BCG) therapy.
The study aims to enroll 37 subjects with confirmed NMIBC and BCG unresponsive disease, which includes carcinoma in situ (CIS) with or without high-grade Ta or T1 papillary disease. Subjects will receive CG0070 administered intravesically following bladder washes with 5% n-dodecyl-B-D-maltoside (DDM) and normal saline. This is followed by intravenous administration of pembrolizumab.
The primary completion date is estimated to be June 2023, with the same date estimated for study completion. The primary outcome measure is the complete response rate at 12 months as defined by FDA guidelines for NMIBC.
Critiques of Study Design: The study is open-label and single-arm, which means there is no placebo control or comparison group. This can introduce biases as both participants and researchers know the treatment being administered, which might affect the reported efficacy and adverse events. Since it is a single-arm trial, the results may not be easily generalizable without comparison to standard therapies or a placebo-controlled group. The sample size of this study (37 subjects) is relatively small, which may limit the statistical power and the ability to detect differences or to generalize the findings to a larger population. Interim analysis or adaptive design methods might not be included, which could help in case of unforeseen safety issues or efficacy concerns. Defining BCG failure as persistent or recurrent disease within 12 months is in-line with current understandings, but may be subjected to varying interpretations depending on prior treatment regimens.
Operational and Technical Challenges: Ensuring patient adherence to the treatment schedule which includes both intravesical and intravenous administrations spanning over 2 years could be challenging. Intravesical administration may come with specific challenges, such as ensuring proper bladder washing technique, potential discomfort for patients, and risk of urinary infections. Monitoring safety and adverse events comprehensively in an open-label study requires stringent operational protocols to minimize observer bias. Ensuring consistent criteria for evaluating complete response across patients and minimizing variability in assessment. Retention of patients over the lengthy study period might be difficult; dropouts could affect the study's power and outcomes. Handling advanced therapies like CG0070 and pembrolizumab may require special logistics, storage, and administration training for the involved healthcare professionals. Ensuring the quality and consistency of the transduction-enhancing agent (DDM) and its administration protocol.
Potential for Proof-of-Concept:The proof-of-concept for using Cretostimogene (CG0070) in combination with pembrolizumab in BCG-unresponsive high-risk non-muscle invasive bladder cancer (NMIBC) rests on demonstrating a reasonable complete response rate, paired with an acceptable safety profile.
Appropriateness of Primary and Secondary Endpoints:Primary Endpoint – The study uses the complete response rate at 12 months as the primary endpoint. This endpoint is appropriate for proof-of-concept as it directly assesses the efficacy of the treatment in eliminating detectable cancer lesions in the short term. It aligns well with clinical objectives, regulatory expectations, and patient outcomes.
Secondary Endpoints – The secondary endpoints including the incidence of adverse events, median duration of response, overall survival, and progression-free survival, complement the primary endpoint and help delineate the safety profile and the durability of the treatment effect, which are critical for the risk-benefit analysis and future larger-scale trials.
Inclusion/Exclusion Criteria Appropriateness:Key Inclusion Criteria – The requirements for the patient's performance status, pathology confirmation, BCG failure within a certain time frame, and refusal or ineligibility for radical cystectomy are critical for selecting a homogenous and relevant patient population. These criteria ensure that the study targets the intended high-risk NMIBC population that requires alternative treatments due to BCG-unresponsiveness.
Adequate BCG therapy definition – Ensuring that patients have received adequate BCG treatments helps in selecting a population that has genuinely failed standard BCG therapy, reinforcing the rationale for considering novel therapies.
Key Exclusion Criteria – The exclusion of immunodeficient individuals, those who have had previous adenovirus-based or checkpoint inhibitor therapies, significant cardiac disease, and active autoimmune disease are measures to protect patient safety and to avoid confounding variables that might obscure the interpretation of the efficacy and safety of the treatment.
Reproducibility Challenges:Inclusion/Exclusion Criteria – The criteria for defining BCG unresponsiveness and BCG treatment adequacy are relatively precise, which is advantageous for reproducibility. However, variability across sites in interpreting these criteria, particularly in patients close to the cutoffs, could present challenges. The specific requirement of BCG treatment schedules and prior assessments within specified time frames may lead to differing applications in a larger multi-center setting.
Further, the exclusion of patients for reasons such as immunodeficiency or autoimmune disease may limit the generalizability of results to all high-risk NMIBC patients, because these individuals may still be part of the broader patient population encountered in practice.
Operational Reproducibility Challenges:- A considerable proportion of eligible patients may choose to undergo radical cystectomy or may not meet the strict organ function requirements, potentially limiting the number of patients available for study participation. Assessing organ function and other exclusion criteria uniformly across sites might be difficult, especially when different laboratories and diagnostic tools are used. Management and monitoring for the exclusion of patients based on prior treatments and health conditions require rigorous medical history documentation and verification.
Overall, while the proof-of-concept potential for Cretostimogene in this setting is strong given the rigor of the primary and secondary endpoints, careful attention should be paid to the uniform application of inclusion and exclusion criteria to enhance the study's reproducibility and internal validity.
Results
-
Response Rates:
- Interim results from the CORE-001 trial show a high rate of efficacy, with 85% (29 out of 34) of the evaluable patients achieving a complete response (CR) at any time following the completion of induction therapy.
-
Durability of Response:
- The responses to cretostimogene appear durable, with 82% (27 out of 33) of evaluable patients maintaining a CR at six months.
- At the 12-month mark, 68% (17 out of 25) of evaluable patients continued to maintain their CR.
-
Safety and Tolerability:
- Cretostimogene was generally well-tolerated, with most adverse events reported being transient, localized, and Grade 1 or 2 in severity, typically related to the urinary tract.
- One Grade 2 serious adverse event (SAE) related to cretostimogene—urinary retention—was reported and resolved.
- There were no discontinuations from the trial deemed related to cretostimogene as of the safety data cutoff.
- In relation to pembrolizumab, a drug used in conjunction with cretostimogene, two Grade 3 SAEs (adrenal insufficiency and immune-mediated hepatitis) were reported, which align with known immune-related adverse events associated with anti-PD1 checkpoint inhibitor (CPI) therapy.
- Four Grade 3 adverse events related to pembrolizumab led to discontinuation, with one patient resuming dosing after missing one dose. Post-cutoff, three more adverse events led to the discontinuation of pembrolizumab.
-
Conclusion:
- The interim clinical data from the CORE-001 trial suggest that cretostimogene, likely being used in combination with pembrolizumab, provides a substantial complete response in the majority of patients with high-risk NMIBC who are unresponsive to BCG therapy.
- Responses have been durable, with a significant proportion of patients retaining a CR for up to a year.
- Cretostimogene's safety profile is favorable, with most cretostimogene-related adverse events being of low grade and transient. Notably, pembrolizumab has been associated with Grade 3 immune-related SAEs and discontinuations, which is consistent with its known safety profile.
PIVOT-006
Summary of Study Design:The Phase 3 clinical trial described is designed to evaluate the efficacy of cretostimogene grenadenorepvec (presumably an oncolytic adenovirus therapy) as an adjuvant treatment for intermediate-risk non-muscle invasive bladder cancer (IR-NMIBC) following a transurethral resection of the bladder tumor (TURBT). This is an open-label, randomized, and parallel-assignment interventional study comparing two arms:
Arm A (Experimental): Participants will receive TURBT followed by adjuvant cretostimogene grenadenorepvec and maintenance doses through Month 13, if there is no disease recurrence. The treatment includes the use of n-dodecyl-B-D-maltoside (DDM) as a transduction-enhancing agent.
Arm B (No Intervention/Observation): Participants will undergo TURBT and then enter a surveillance phase. If these participants experience a recurrence of IR-NMIBC, they will be offered cretostimogene treatment as per the schedule in Arm A (Extension Arm).
The primary outcome measure is Recurrence Free Survival (RFS) at 51 months post-TURBT. Secondary outcome measures include RFS at 12 and 24 months, and the incidence of adverse events over a 52-month period.
The study aims to enroll approximately 426 participants, with an estimated study completion date of January 2030.
Critiques of the Study Design:- Having a control arm (observation) allows for comparison against the standard practice of monitoring after TURBT without additional adjuvant therapy, helping to establish the incremental benefit of cretostimogene.- The study is open-label, which could introduce bias since participants and researchers know which treatment is given. Blinding this type of study could be challenging due to the nature of the intervention.- The large sample size for a Phase 3 trial is appropriate for providing adequate statistical power to detect a difference in RFS between the two arms.- Long follow-up period (51 months) is conducive to evaluating the long-term efficacy of adjuvant treatment in preventing recurrence.- Offering cretostimogene to participants in the observation arm after recurrence, while ethical and beneficial for those patients, may complicate the analysis of long-term outcomes.
Operational and Technical Challenges:- The recruitment of a sufficient number of participants who meet the specific criteria for IR-NMIBC and the management of a large number of study sites and subjects over a long period will be challenging.- The administration of an oncolytic virus requires specialized handling, infrastructure, and patient monitoring to manage potential side effects associated with both the virus and the DDM used.- Ensuring consistent adherence to the protocol across all sites, especially considering the complexity of the intervention and the length of the follow-up.- Retention of participants for the full duration of the trial is crucial for maintaining study power. Dropouts or missing data over such a long time frame could affect the integrity of the outcome measures.- Potential crossover of patients from the observation arm to the experimental treatment after recurrence could influence the interpretation of long-term efficacy and safety results.
Overall, the Phase 3 trial design seems adequately powered and well-structured to test the efficacy and safety of cretostimogene grenadenorepvec as adjuvant therapy post-TURBT in IR-NMIBC. The inclusion of a control arm for comparison and the robust follow-up times are positive design aspects that are likely to provide valuable insights into the long-term benefits and risks associated with the treatment. However, the open-label design, operational complexities, and potential for patient crossovers must be carefully managed to ensure the integrity and reliability of the study's results.
Appropriateness of Primary and Secondary Endpoints:The primary endpoint of Recurrence-Free Survival (RFS) at 51 months post-TURBT is appropriate and ambitious, providing long-term data on the efficacy of Cretostimogene to prevent recurrence. Secondary outcomes, such as RFS at 12 and 24 months and the incidence of adverse events, will provide additional benchmarks for efficacy and safety at earlier time points, which are important for clinical decision-making and regulatory approval processes.
Inclusion/Exclusion Criteria:The inclusion criteria are comprehensive and align with standard definitions of intermediate-risk disease, which helps to ensure a homogenous study population. The specific numerical and categorical definitions (e.g., tumor size, grade, multifocality, recurrence frequency) enhance clarity and appropriateness of participant selection. The requirement for acceptable baseline organ function is standard for clinical trials to ensure patient safety.
The exclusion criteria are stringent to filter out patients at higher risk (e.g., those with high-grade T1 tumors or carcinoma in situ) and at lower risk (e.g., solitary low-grade Ta ≤ 3 cm tumors) who have different standard-of-care treatments and prognosis. Additionally, excluding patients with prior adenovirus therapy is prudent to avoid confounding immune responses that could skew the effectiveness or safety profile of the investigational treatment.
Perhaps the most significant reproducibility challenge stems from the criteria that delineate intermediate risk, such as tumor size and grading, which are subject to inter-observer variation. Even with pathologically confirmed IR-NMIBC, assessments could differ between pathologists or institutions, potentially leading to variability in patient eligibility. Moreover, excluding patients with disease in the prostatic urethra or the upper genitourinary tract within 24 months of randomization may depend on the meticulousness of patient medical record reviews and could be challenging to standardize across sites.
It is essential to ensure that the criteria for IR-NMIBC are applied consistently across study sites to maintain the internal validity of the trial. Standardized training for site investigators and pathologists, use ofcentralized pathology review, or clear imaging and surgical documentation guidelines could be implemented to mitigate these challenges.
Overall, the study design incorporating stringent inclusion/exclusion criteria stands to provide a strong proof-of-concept for Cretostimogene in the targeted patient population. While challenges related to patient selection reproducibility exist, they are not insurmountable and should be addressed through careful study execution and quality control measures.
Weekly analyses of biotech startups, generated by AI
Receive high quality, AI-generated analyses of biotech startups, public companies and scientific papers each week.
Market overview
BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer
To evaluate the market opportunity in BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer (HR NMIBC), it's essential to understand the current treatment landscape, existing competitors, standard of care, and the unmet medical need.
With over 82,000 new cases of bladder cancer and a total of approximately 725,000 people living with the disease in the United States, the patient population is significant. Moreover, the high recurrence rate post-treatment in NMIBC, which ranges from 15% to 61% within one year and 31% to 78% within five years, highlights a large and ongoing need for effective treatments.
For patients with NMIBC, especially high-risk categories, the standard of care typically starts with transurethral resection of bladder tumor (TURBT) followed by intravesical therapy with Bacillus Calmette-Guérin (BCG), which is a form of immunotherapy. BCG is the most effective treatment for reducing tumor recurrence and progression. However, there is a significant unmet medical need as BCG-unresponsive HR NMIBC poses a treatment challenge. A significant portion of patients do not respond to BCG or relapse after initial response. For these patients, the next option if they are unfit for or refuse cystectomy can be limited.
There has been significant interest in developing therapies for BCG-unresponsive NMIBC. Valrubicin is one existing therapy but has limited efficacy. Pembrolizumab (Keytruda) received accelerated approval by the FDA specifically for the treatment of BCG-unresponsive, high-risk NMIBC with CIS with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. Other checkpoint inhibitors and novel agents are being investigated.
The landscape for BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer (HR NMIBC) sees ongoing research and development efforts, focusing on the need for alternative treatments due to BCG’s limitations. Several therapeutic approaches are promising contenders and could potentially compete with Cretostimogene:
1. Pembrolizumab (Keytruda): An FDA-approved immunotherapy for patients with BCG-unresponsive NMIBC who are not candidates for cystectomy. As a PD-1 inhibitor, it represents one of the successes in the field of bladder cancer immunotherapy.
2. Nivolumab (Opdivo): Another PD-1 inhibitor being evaluated for use in BCG-unresponsive bladder cancer, with studies demonstrating potential benefits similar to pembrolizumab.
3. Atezolizumab (Tecentriq) and Durvalumab (Imfinzi): Both are PD-L1 inhibitors that have shown promise in bladder cancer and are in various stages of clinical development for NMIBC.
4. Oportuzumab Monatox (Vicineum): Developed by Sesen Bio, this is a fusion protein designed to target epithelial cell adhesion molecule (EpCAM) antigens on the surface of tumor cells and deliver a potent toxin to induce cell death. It's specifically aimed at BCG-unresponsive NMIBC.
6. Intravesical Gemcitabine and Docetaxel: While not novel systemic agents, these chemotherapeutic drugs have been used intravesically with some success in BCG-unresponsive NMIBC and are part of ongoing investigations.
7. Nadofaragene firadenovec (rAd-IFN/Syn3): A novel gene therapy being developed that delivers interferon-alpha directly into the bladder wall, made by FKD Therapies and licensed to Ferring Pharmaceuticals.
8. ALT-803: A complex of an interleukin-15 superagonist and a dimeric IL-15 receptor alpha sushi domain, developed by Altor BioScience Corporation, which could enhance the activity of NK and T cells against bladder cancer cells.
9. Mitomycin C Gel (UGN-101): Developed by UroGen Pharma, this is a gel formulation of the chemotherapy agent Mitomycin C, which can be administered via catheter into the bladder, potentially targeting tumor cells directly.
Considering the vast array of treatments in the pipeline, it’s clear that the space for BCG-unresponsive HR NMIBC treatments is highly competitive. In this environment, factors such as efficacy, safety, ease of administration, cost, and the production capacity to meet demand are critical to a new therapy's success. Strategies involving combination therapies, unique mechanisms of action, or improved delivery methods are avenues that various competitors are exploring.
As each of these prospective therapies progresses through clinical development, their comparative performance in clinical trials in terms of cancer recurrence rates, progression-free survival, overall survival, and quality of life improvements will significantly shape the treatment landscape. Companies with strong partnerships, adequate funding, and effective go-to-market strategies will be well-positioned to capitalize on this opportunity provided they can deliver on clinical outcomes and demonstrate value in the complex healthcare environment. Cretostimogene's ultimate competitive position in this field will rely on its differentiation from these other therapies in development, both in terms of clinical outcomes and market access.
Intermediate-Risk Non-Muscle Invasive Bladder Cancer
BCG-exposed and BCG-naive non-muscle invasive bladder cancer (NMIBC) represents a heterogeneous group of patients with a variable prognosis, and understanding their treatment landscape is crucial for determining the market opportunity for new therapies like Cretostimogene.
Standard of Care for Intermediate-Risk NMIBC:The current standard of care for intermediate-risk NMIBC typically includes transurethral resection of the bladder tumor (TURBT) followed by intravesical therapy. Intravesical therapy may involve chemotherapy (such as mitomycin C) or immunotherapy (such as BCG), aimed at reducing the risk of recurrence and progression. While intermediate-risk patients may receive less intensive BCG treatment compared to high-risk patients, the overall management is aimed at preventing progression while limiting treatment-related side effects.
Unmet Medical Need in Intermediate-Risk NMIBC:While patients classified as intermediate-risk have a lower risk of progression compared to high-risk patients, they still have a significant chance of recurrence. The optimal management, in terms of intravesical therapy intensity and duration, remains a subject of clinical investigation.
Moreover, not all patients can tolerate BCG, and some may have contraindications to its use, creating a need for alternative treatments. In cases where patients are intolerant or have relapsed after BCG and chemotherapy, there is a clear unmet need for new, effective therapies that can prevent progression to muscle-invasive disease while preserving the bladder.
Market Opportunity for Cretostimogene:Given the estimated market size of $9.9 billion for bladder cancer treatments by 2028, combined with the incidence and prevalence rates, there is a significant market opportunity for new treatments. For Cretostimogene to capture a portion of this market in intermediate-risk NMIBC, it would need to address several points:
Demonstrate Superior Efficacy: Cretostimogene should prove superior or at least non-inferior to existing treatments in reducing recurrence rates, based on rigorous clinical trials.
Offer Safe and Well-tolerated Option: It must be safe and well-tolerated, especially considering the demographic commonly affected by bladder cancer, which includes older patients who may have concomitant health issues.
Possess Favorable Pharmacoeconomics: The drug should offer a clear cost-benefit ratio compared to current therapies, taking into account direct and indirect healthcare costs associated with bladder cancer recurrences and progression.
Receive Endorsement from Professional Bodies: Professional endorsements and inclusion in treatment guidelines would support its adoption by the urological community.
Insurance Coverage: Securing broad coverage from payers would be essential for patient access to Cretostimogene.
Other successful drugs in the indication for NMIBC don't typically differentiate between intermediate and high-risk in their indications but often rely on risk stratification to inform treatment. For Cretostimogene, if it can differentiate itself as an especially effective option for those intermediate-risk patients who are not adequately managed by current treatments, it can become a valuable asset in the NMIBC treatment arsenal.
Competitive Landscape: In the realm of intermediate-risk non-muscle invasive bladder cancer (NMIBC), several promising treatments are in development that could potentially compete with a hypothetical product such as Cretostimogene. The competitive landscape is shaped by a number of factors, including the efficacy and safety profiles of the treatments, their convenience of administration, and ultimately their cost. Here are some categories of promising treatments that are under development:
1. Enhanced Intravesical Therapies:These are therapies administered directly into the bladder. Developers are working on novel formulations of existing chemotherapeutics, such as mitomycin C, optimized for better efficacy (e.g., compounded with a gel for prolonged exposure). UroGen Pharma's mitomycin gel (Jelmyto) is one such product designed for easier and more effective intravesical administration.
2. Immunotherapy Enhancements:New formulations or combinations of BCG are being researched to enhance its efficacy and reduce side effects. Additionally, there's keen interest in other immunomodulatory agents that might work synergistically with BCG or as monotherapy to recruit immune cells to target bladder cancer cells.
3. Gene Therapies:Gene therapy candidates are being pursued, which involve the delivery of genetic material (e.g., using viral vectors) into bladder cells to induce a therapeutic effect. An example is nadofaragene firadenovec (Instiladrin), which delivers an interferon gene to bladder cells.
4. Checkpoint Inhibitors:Drugs that inhibit immune checkpoints like PD-1/PD-L1 may also be evaluated for intermediate-risk NMIBC. Current checkpoint inhibitors like pembrolizumab and atezolizumab are predominantly used in higher-risk or metastatic settings, but their role could expand to earlier stages of the disease.
5. Novel Targeted Agents:Molecular characterization of bladder tumors may reveal targets for therapy. For instance, fibroblast growth factor receptor (FGFR) inhibitors like erdafitinib have been approved for metastatic bladder cancer and might be evaluated for non-muscle invasive forms based on tumor genetics.
6. Device-Assisted Therapies:New medical devices and techniques are being utilized to enhance the delivery of intravesical agents, for example, using hyperthermia or electromotive drug administration to increase the uptake of chemotherapeutics.
7. Oncolytic Viruses:Viruses engineered to infect and kill cancer cells selectively are in clinical trials. CG0070 is an example of an oncolytic virus that has a dual mechanism of action as both a selective killer of cancer cells and as an immunotherapy.
8. Combination Therapies:Trials combining agents with different mechanisms, such as a chemotherapeutic and an immunotherapy, are also underway, aiming to offer a one-two punch against bladder cancer.
9. Novel Drug Classes:Other classes of drugs, such as antisense oligonucleotides or siRNA-based therapies that target the RNA molecules to inhibit the expression of specific cancer-related proteins, might also figure in the future of NMIBC treatment.
BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer in combination with pembrolizumab
Given the context of Non-Muscle Invasive Bladder Cancer (NMIBC), Cretostimogene offers a potential treatment for a condition with a high recurrence rate and an unmet need for more effective therapies, especially in the BCG-exposed and BCG-naive populations.
Standard of Care:The current standard of care for NMIBC includes transurethral resection of the bladder tumor (TURBT), followed by intravesical therapies. The intravesical administration of Bacillus Calmette-Guérin (BCG) is a common treatment for high-risk NMIBC to reduce recurrence and prevent progression. However, BCG therapy has associated issues, such as BCG shortage, BCG intolerance, and a significant portion of patients who do not respond or whose disease recurs after BCG treatment (BCG-unresponsive).
BCG-Exposed Population:BCG-exposed patients are those who have received BCG therapy but either did not respond to the treatment or experienced relapse after an initial response. This group has a critical need for additional treatment options because the standard BCG therapy has already failed them.
BCG-Naive Population:BCG-naive patients are those who have never received BCG – either because they are newly diagnosed or because they have been managing their disease through other, potentially less effective, modalities. For these patients, the challenge is to identify treatment options that are as effective or more effective than BCG and to provide alternatives for BCG in cases of shortage or for patients for whom BCG is not recommended due to medical reasons.
Market Opportunity:Given the prevalence and recurrence rates of NMIBC as outlined, the market demand for new therapies in both BCG-exposed and BCG-naive populations is substantial. The global bladder cancer treatment market, estimated to reach $9.9 billion by 2028, provides a significant opportunity for new drugs like Cretostimogene.
The key for Cretostimogene would be to demonstrate its ability to reduce recurrence rates and address the considerable unmet clinical need, especially in BCG-unresponsive patients, where current options are limited and may lead to radical interventions such as cystectomy. Furthermore, in BCG-naive patients, if Cretostimogene can show benefit comparable to or better than BCG, it may be a preferred option, especially during BCG shortages or for patients unable to undergo BCG treatment.
Revenue build and model
BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer in combination with Pembrolizumab
Based on the extensive information provided, we can construct a hypothetical revenue build for Cretostimogene in BCG-unresponsive high-risk non-muscle invasive bladder cancer (HR NMIBC) in combination with pembrolizumab. The following are plausible estimates to illustrate how the financial model might be structured. Please note that actual values would require in-depth market analysis and access to proprietary information from the company developing Cretostimogene. These placeholder estimates are for illustrative purposes only.
1. Access to Treatment: - Target population: Assume the total addressable market for HR NMIBC is 25,000 patients annually in the United States (Placeholder estimate based on market research). - Market penetration rate: 5% in Year 1, growing to 25% in Year 5. This takes into account the time needed for market adoption and physician awareness.
2. Gross-to-Net Adjustments: - List price: $150,000 per annual course of treatment (based on prices of similar high-cost cancer therapies). - Gross-to-net discount: Assuming a 30% discount due to negotiations with payers, rebates, and patient assistance programs.
3. Insurance Coverage: - Coverage rate: Assuming 90% of patients have insurance coverage, as treatment is for a life-threatening condition where coverage is typically expected.
4. Duration of Therapy: - Course of therapy: Assume an average of 1 year of treatment, which would typically include induction and maintenance phases.
Please note, this is a simplified model and in practice, factors such as regional pricing variations, international market expansion, competition, and changes in clinical guidelines would further affect the revenue build. Additionally, real-world data collection and post-marketing surveillance costs, as well as opportunities like label expansions, could significantly alter this model.
To estimate the probability of Phase 3 and FDA submission success for Cretostimogene in BCG-unresponsive high-risk non-muscle invasive bladder cancer (HR NMIBC), given the industry standard clinical trial success rates and the positive interim clinical data provided, we can modify the base rates using Bayesian methodology. The Bayesian approach updates the prior probability (industry success rates) based on new evidence (interim Phase 2 data) to generate a posterior probability reflecting the updated chances of success.
Industry Standard Success Rates:
- Phase 2 to Phase 3 success rate: 24.6%
- Phase 3 to FDA submission success rate: 47.7%
Positive Interim Phase 2 Data Analysis:
- High Complete Response Rates: The high CR rates after induction therapy and the durability of the response over 6 and 12 months suggest strong efficacy, which can be considered favorably relative to typical Phase 2 outcomes.
- Safety Profile: The generally well-tolerated nature of Cretostimogene and manageable adverse events might also speak to the plausibility of success moving into later phases of clinical trials. However, Grade 3 SAEs related to pembrolizumab underline the importance of continued vigilance in safety monitoring.
Adjusted Probability Estimates:
-
Phase 2 Success: Given the strong interim results, we could optimistically assume the probability of Phase 2 success might be higher than the industry average. If we assume the positive data can double the industry standard probability (which is a significant uplift), we might estimate a (24.6% times 2) or 49.2% chance of Phase 2 success. This assumes that the trial design and endpoints are well-aligned with regulatory expectations and that the high response rates are sustained.
-
Phase 3 Success: Given the positive efficacy and safety data and the high unmet medical need in BCG-unresponsive HR NMIBC, we could increase the probability of success by 10%, yielding a 52.5%. We use a lower adjustment because the Phase 2 adjustment reflects positive interim results, which justifies a higher increase in probability of success.
It’s important to note that these are hypothetical adjustments and actual probabilities could be significantly different. Real-world probabilities are inherently uncertain and influenced by numerous variables, including the precise nature of the clinical trial results, changes in the competitive landscape, regulatory precedents, the disease's prevalence and impact, and the specifics of the drug under consideration.
Finally, regulatory agencies like the FDA consider a broad range of data beyond efficacy, such as the severity of side effects, the drug's mechanism of action, the overall treatment landscape, and the specifics of drug administration. Therefore, each drug’s journey through clinical trials and the regulatory process is unique, and estimates should be treated as educated guesswork pending more definitive data.
Intermediate-Risk Non-Muscle Invasive Bladder Cancer
Creating a hypothetical revenue build for Cretostimogene in Intermediate-Risk Non-Muscle Invasive Bladder Cancer (NMIBC), where the patient population does not overlap with high-risk NMIBC for which it is combined with pembrolizumab, would involve estimating the potential market size, treatment cost, insurance coverage, and other financial factors. The following are purely hypothetical placeholder estimates meant to illustrate the process of constructing a revenue model.Total Addressable Market (TAM): Estimate the number of intermediate-risk NMIBC patients annually. Placeholder Estimate: 30,000 patients/year in the US.
Market Penetration Rate: Estimate the percent of TAM that Cretostimogene captures annually. Placeholder Estimate: 5% Year 1, increasing by 5% annually to 20% by Year 5.
Insurance Coverage: Estimate the percent of patients with insurance coverage for Cretostimogene treatment. Placeholder Estimate: 90%.
Duration of Therapy: Estimate based on the typical course of treatment for bladder cancer. Placeholder Estimate: 1 year of therapy per patient.
Please note that the revenue model is ultimately subject to real-world data from commercialization efforts, competitive dynamics, and evolving market conditions. Additionally, the model may need to be adjusted based on global expansion and penetration in markets outside the US.
Platform overview
CG Oncology's scientific strategy is centered around the development of innovative therapeutics, specifically using oncolytic viruses, to treat cancer with an initial focus on non-muscle invasive bladder cancer (NMIBC). Oncolytic viruses are genetically modified viruses that selectively infect and destroy cancer cells while stimulating a systemic immune response against the tumor. Cretostimogene, CG Oncology's lead product candidate, appears to be an oncolytic virus therapy based on the description provided.
Overview of CG Oncology's Scientific Strategy:
Phase 3 BOND-003 Trial for Cretostimogene Monotherapy in High-Risk BCG-Unresponsive NMIBC: CG Oncology aims to complete its Phase 3 trial and subsequently pursue FDA approval based on the guidance that single-arm trials with complete response (CR) rate as a primary endpoint, considering duration of response (DOR), may be appropriate for approval.
Expansion into Additional NMIBC Indications: The strategy also includes expanding the use of cretostimogene to other types of bladder cancer, specifically in intermediate-risk NMIBC and high-risk BCG-exposed/BCG-naïve NMIBC. This is intended to not only serve a broader patient population but also address the global BCG shortage issue.
Combination Trials with Checkpoint Inhibitors (CPIs): In addition to monotherapy, CG Oncology is also evaluating the efficacy of cretostimogene in combination with other immunotherapies like pembrolizumab and nivolumab. Combining oncolytic viruses with CPIs is a known immunotherapeutic strategy that may potentially increase anti-tumor activity.
Commercialization and Marketing Strategy: If cretostimogene is approved by the FDA, CG Oncology is preparing to market and commercialize the product within the United States, building on in-house capabilities and potentially leveraging the practice of intravesical (IVE) delivery, which is familiar to urologists due to BCG therapy.
Manufacturing and Supply Chain Expertise (CMC): The company is also focusing on Chemistry, Manufacturing, and Controls (CMC) expertise, aiming to establish a high-yield manufacturing process that can scale rapidly to meet demand through a strategic mix of in-house knowledge and external partnerships.
Similar Approaches:Other companies, such as Amgen with talimogene laherparepvec (T-VEC) for melanoma, have developed oncolytic virus therapies that are FDA-approved. The combined approach of using oncolytic viruses with CPIs has been explored in various cancers due to the potential synergistic effects of these treatments.
Risks and Pitfalls:The approach of using oncolytic viruses, while promising, has several risks and challenges:
Clinical Efficacy: The efficacy of cretostimogene needs to be conclusively demonstrated in ongoing and future trials.
Safety Profile: Although no grade 4 or 5 treatment-related adverse events (TRAEs) have been observed thus far, the long-term safety profile is critical for FDA approval and broader patient acceptance.
Regulatory Risks: Single-arm trials are sometimes considered less definitive than randomized controlled trials, and there could be regulatory challenges unless the results are robust and convincing.
Market Acceptance and Commercialization: The commercial success will depend on the product's adoption by healthcare providers, reimbursement by payers, and patient preference, especially in comparison to existing therapies.
Manufacturing Challenges: Oncolytic viruses can be complex to manufacture at scale, and maintaining quality control and cost-effectiveness are critical for commercial viability.
Competition: The market for cancer therapeutics is highly competitive, with ongoing research and new therapeutics continually emerging.
In conclusion, CG Oncology's strategy is consistent with an emerging trend in cancer therapeutics of targeting multiple facets of NMIBC treatment, both as a monotherapy and in combination with other immune modulators. While there are inherent risks, the approach is promising and could potentially bring new treatment options to patients suffering from various forms of bladder cancer.