Contineum Therapeutics IPO investment analysis
March 20, 2024
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or an ongoing business relationship with the company.
Overview
Contineum Therapeutics is a clinical stage biopharmaceutical company developing novel orally administered small molecule therapies targeting neuroinflammation and immune (NI&I) indications where there is a significant unmet medical need. By focusing on specific molecular pathways associated with clinical impairments, the company has engineered a portfolio of distinctive small molecule drug candidates intended to fundamentally alter disease progression.
Product Pipeline:
- PIPE-791: This leading asset is an innovative brain-penetrant small molecule inhibiting LPA1R, currently in development for Idiopathic Pulmonary Fibrosis (IPF) and Progressive Multiple Sclerosis (MS). Preliminary studies indicate PIPE-791's high bioavailability, low plasma protein binding, and extended receptor occupancy, potentially positioning it as a superior therapy in its class.
- PIPE-307: Another key candidate, PIPE-307, is a novel selective muscarinic type 1 (M1R) antagonist in development for depression and Relapsing-Remitting MS (RRMS), representing significant progress as potentially the most clinically advanced selective M1R antagonist.
The company has established a development collaboration with J&J for PIPE-307, enhancing its commercialization prospects. It is also progressing in preclinical studies for additional molecules, including CTX-343, a peripherally-restricted LPA1R antagonist, targeting other NI&I indications.
Contineum has successfully completed Phase 1 trials for both PIPE-791 and PIPE-307, showing safety, tolerability, and favorable pharmacokinetics. It plans to submit a Clinical Trial Application (CTA) to the MHRA for a Phase 1b trial of PIPE-791 to refine dose-selection based on PET imaging for subsequent Phase 2 trials targeting IPF and Progressive MS in 2024.
Market Potential and Unmet Needs:
- IPF: With an estimated 130,000 cases in the US and three million globally, IPF represents a lucrative market. Current treatments do not halt disease progression and have significant side effects, highlighting a substantial unmet need that PIPE-791 aims to address.
- Progressive MS and RRMS: MS, especially in its progressive forms, lacks sufficient treatment options, marking a high-value domain for PIPE-791 and PIPE-307.
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| PIPE-791 | Small molecule | LPA1R Antagonist | Idiopathic pulmonary fibrosis | |||||||
| PIPE-791 | Small molecule | LPA1R Antagonist | Progressive multiple sclerosis | |||||||
| CTX-343 | Small molecule | LPA1R Antagonist | Undisclosed | |||||||
| PIPE-307 | Small molecule | M1R Antagonist | Relapse remitting multiple sclerosis | |||||||
| PIPE-307 | Small molecule | M1R Antagonist | Depression |
Highlights and risks
LPA1R inhibitors have shown promise in IPF and PIPE-791 has best-in-class potential
Significant unmet need in IPF and progressive MS creates significant market potential
Preclinical data, as well as comparison with Clemastine, suggests PIPE-307 has potential remyelinating capabilities
Limited or no direct clinical evidence supporting effectiveness of M1R antagonism in MS or depression
Targeting competitive indications where clear differentiation is required for significant market share
Phase 1 studies show encouraging safety profiles, but safety issues have hindered other agents in targeted indications
Valuation
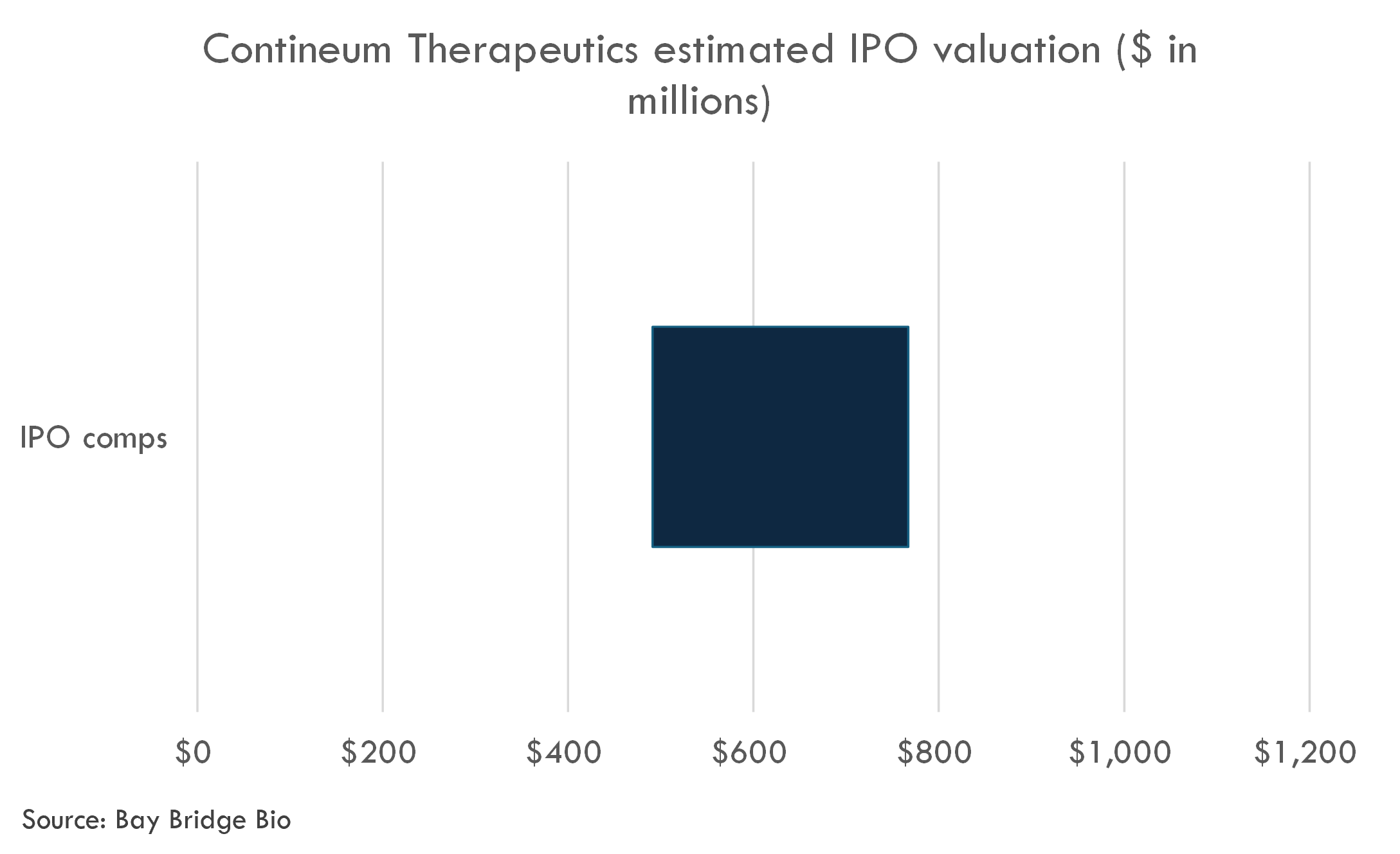
The company's last private financing round, a $140M Series C from February 2021 until August 2023, valued the company at an estimated post-money valuation of $306 million.
The company filed to go public in March 2024. The fully-diluted IPO post-money valuation is likely to be in the range of $491-767 million.
PIPE-791
Scientific background
Idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis (MS) are chronic diseases characterized by progressive fibrosis and neurodegeneration, respectively. Both conditions lack curative treatments, and therapies that can significantly slow their progression are in high demand. A lysophosphatidic acid receptor 1 (LPA1R) antagonist represents a novel therapeutic approach to both IPF and progressive MS, based on the understanding of LPA1R's role in pathophysiology.
Therapeutic Rationale in Idiopathic Pulmonary Fibrosis (IPF)
- Fibroblast Activation and Proliferation: LPA acts through its receptor, LPA1R, to stimulate the activation and proliferation of fibroblasts. These cells are key contributors to the excessive deposition of extracellular matrix proteins, leading to the thickening and scarring of lung tissue that characterizes IPF. By blocking LPA1R, it is hypothesized that the antagonist could prevent fibroblast activation, thereby halting or slowing the fibrotic process.
- Inflammation and Immune Response Modulation: LPA is involved in the modulation of immune responses and inflammation. Given that chronic inflammation is believed to precede fibrosis in IPF, targeting LPA1R could also help in reducing inflammatory responses, thus indirectly contributing to the control of fibrotic progression.
Therapeutic Rationale in Progressive Multiple Sclerosis (MS)
- Neuroinflammation and Demyelination: LPA signaling through LPA1R has been implicated in the neuroinflammatory processes and the demyelination observed in MS. Blocking LPA1R could potentially reduce neuroinflammation and protect against myelin and neuronal damage.
- Neuroprotection: LPA1R antagonism may offer neuroprotective effects by mitigating glial activation (astrocytes and microglia), which contributes to neuronal damage and the progression of disability in progressive MS. The reduction in neuroinflammation and the neuroprotective effect could slow disease progression and alleviate symptoms.
- Barrier Integrity: LPA has been shown to compromise the integrity of the blood-brain barrier (BBB), facilitating the infiltration of immune cells into the central nervous system (CNS) and exacerbating neuroinflammation in MS. An LPA1R antagonist could help in maintaining BBB integrity, thus potentially reducing pathological immune cell infiltration and its associated damage.
In conclusion, by targeting LPA1R, an antagonist could interrupt key pathophysiological processes involved in both IPF and progressive MS, offering a therapeutic approach that could slow disease progression and ameliorate symptoms in these debilitating conditions. This therapeutic strategy is underpinned by the central roles that LPA signaling plays in fibrosis, inflammation, neurodegeneration, and neuroinflammation, making LPA1R a promising target for intervention in these diseases.
The science described in the response is rooted in a growing body of preclinical and some early clinical evidence but remains an area of active research with several aspects subject to ongoing investigation and debate.
Established Science and Evidence
- Role of LPA and LPA1R in Fibrosis and Inflammation: The involvement of lysophosphatidic acid (LPA) in fibrosis and inflammation is well-established in the scientific literature, largely based on preclinical studies. The mechanism by which LPA acts through its receptor LPA1R to promote fibroblast activation and proliferation is supported by cellular and animal models.
- LPA Signaling in Neuroinflammation: There is a solid foundation of preclinical evidence showing that LPA signaling, particularly through LPA1R, contributes to neuroinflammatory processes, which are relevant for diseases like multiple sclerosis.
Areas of Uncertainty and Debate
- Translational Gaps: While animal models have been instrumental in elucidating the role of LPA1R in disease, translating these findings to human disease remains challenging. The complexity of human diseases like IPF and progressive MS means that pathways observed in animal models may not fully replicate human pathophysiology.
- Optimal Targeting and Timing: There is ongoing debate about how best to target LPA1R—whether direct antagonism, modulation of LPA levels, or targeting downstream signaling pathways might be most effective. Additionally, the optimal timing for intervention in the disease course is not yet clear, especially for progressive diseases that have a long preclinical phase.
- Safety and Specificity: The systemic inhibition of LPA1R could potentially have off-target effects or impact physiological processes where LPA signaling plays a protective role. This raises concerns about the long-term safety and specificity of LPA1R antagonists, which are currently areas of active investigation.
Overall Level of Evidence
The mechanistic understanding of LPA1R’s involvement in fibrosis, neuroinflammation, and related pathologies is supported by a substantial body of cellular and animal research, indicating a strong rationale for targeting this pathway. However, the overall level of evidence, particularly from human clinical trials, is less robust at this stage. Most of the evidence supporting the therapeutic potential of LPA1R antagonists comes from preclinical studies, with a limited number of early-phase clinical trials attempting to translate these findings into therapeutic interventions for humans. These trials are crucial for establishing the clinical efficacy and safety profile of LPA1R antagonists in IPF, progressive MS, and other conditions.
In summary, while the scientific rationale for targeting LPA1R in diseases like IPF and progressive MS is compelling and grounded in established science, there remain significant gaps in translating this knowledge into effective therapies. The field is evolving, with ongoing research aimed at addressing these uncertainties and establishing a robust evidence base for clinical applications.
The scientific literature supports the involvement of LPA1R in idiopathic pulmonary fibrosis (IPF) and provides emerging evidence for its role in the pathogenesis of progressive multiple sclerosis (MS), though more robust clinical data in MS is still needed. Here is a summary of the findings from key studies and reviews:
Idiopathic Pulmonary Fibrosis (IPF)
- Preclinical Evidence: A landmark study published in the "Journal of Clinical Investigation" demonstrated that inhibition of LPA1R significantly reduced fibrosis in a mouse model of lung injury. This study provided direct evidence of LPA1R's role in fibrogenesis and its potential as a therapeutic target in IPF (Tager AM et al., 2008).
- Mechanistic Insights: Further research has elucidated the mechanism by which LPA signaling through LPA1R promotes fibroblast activation, proliferation, and differentiation into myofibroblasts, which are key effector cells in the fibrotic process. LPA1R signaling has been shown to mediate the recruitment of fibroblasts and the secretion of profibrotic cytokines, contributing to the pathological tissue remodeling observed in IPF.
- Clinical Correlates: Elevated levels of LPA have been detected in the bronchoalveolar lavage fluid of IPF patients, correlating with disease severity. This supports the involvement of LPA-LPA1R signaling in the human disease process, although direct clinical evidence for the efficacy of LPA1R antagonists in IPF patients is still emerging.
Progressive Multiple Sclerosis
- Pathophysiological Role: The role of LPA1R in progressive MS is based on its contribution to neuroinflammation, neurodegeneration, and demyelination, which are hallmarks of MS pathology. Although direct studies on progressive MS are less plentiful than for IPF, LPA signaling has been implicated in the regulation of immune cell infiltration into the central nervous system and in the activation of astrocytes and microglia, which play a role in the progression of MS.
- Emerging Evidence: Several studies have suggested that LPA and its receptors are elevated in the central nervous system in models of MS, pointing to a potential role in disease progression. For instance, research has indicated that blocking LPA signaling can reduce neuroinflammation and demyelination in animal models of MS, supporting the therapeutic potential of LPA1R antagonism.
While this overview provides a glimpse into the evidence supporting the role of LPA1R in IPF and progressive MS, it's important to note that most of the robust data comes from preclinical studies, especially for progressive MS. The translation of these findings into clinical benefits for patients is subject to ongoing research. Clinical trials designed to test the safety and efficacy of LPA1R antagonists in these diseases will be crucial for validating the therapeutic potential suggested by preclinical models.
The therapeutic rationale for targeting LPA1R in idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis (MS) is underpinned by a mix of preclinical and, to a lesser extent, early clinical studies. Here's a detailed look at the strengths and weaknesses of this evidence base.
Strengths
- Consistency Across Preclinical Models: A significant strength is the consistent observation across various animal models that LPA1R signaling plays a crucial role in fibrosis and neuroinflammation, key pathophysiological processes in IPF and MS, respectively. This body of evidence suggests that LPA1R is a valid therapeutic target.
- Molecular Mechanisms: The molecular mechanisms underlying LPA1R-mediated effects have been relatively well-characterized, offering specific insight into how LPA1R antagonism might exert therapeutic effects. Understanding these mechanisms helps in designing targeted therapies and might aid in predicting efficacy and potential side effects.
- Biomarker Correlation: Elevated levels of LPA and the expression of LPA1R have been correlated with disease severity and progression in patients, providing a biological plausibility to the therapeutic approach. This correlation not only strengthens the rationale but may also facilitate the identification of patient subgroups most likely to benefit from LPA1R antagonism.
Weaknesses
- Translation to Humans: A major weakness is the uncertain translatability of animal model findings to human disease. Differences in disease complexity, as well as in the immune and fibrotic responses between humans and animal models, may limit the applicability of the preclinical data.
- Clinical Evidence Gap: Despite promising preclinical data, there is a relative paucity of robust clinical trial data demonstrating the efficacy and safety of LPA1R antagonists in humans. Early-phase clinical trials have commenced, but large-scale, late-stage clinical trials are needed to confirm the therapeutic value of LPA1R antagonism in IPF and progressive MS.
- Potential Off-Target Effects: The systemic inhibition of LPA1R could lead to unintended off-target effects or disrupt physiological processes where LPA signaling is beneficial. The comprehensive safety profile of LPA1R antagonists remains to be fully characterized, particularly with long-term use.
- Complexity of Disease Pathogenesis: Both IPF and progressive MS are diseases with multifactorial etiologies and complex pathogenesis. Targeting a single receptor, such as LPA1R, may not be sufficient to significantly alter disease progression, especially in advanced stages. This complexity necessitates combination therapies, which introduce additional layers of investigation and potential drug interactions.
In summary, while the evidence base provides a compelling scientific rationale for targeting LPA1R in IPF and progressive MS, there remains a significant need to bridge the gap between preclinical promise and clinical proof of concept. Future research efforts, particularly those focused on comprehensive clinical trials, will be critical in determining the true therapeutic potential of LPA1R antagonists in these challenging diseases.
Competition
The company faces competitors across all of its programs. Key competitors are outlined below, and will be discussed in more detail in the product-specific sections.
| Product Name | Indication | Competing Companies | Competing Products (approved) |
|---|---|---|---|
| PIPE-791 | Idiopathic pulmonary fibrosis | Genentech/Roche, Boehringer Ingelheim, Bristol-Meyers Squibb, AbbVie Inc., Horizon Therapeutics plc, Structure Therapeutics Inc, Roche Holding AG, United Therapeutics Corporation, Pliant Therapeutics, RedX Pharma, Endeavor Biomedicines | Esbriet, Ofev |
| PIPE-791 | Progressive multiple sclerosis | Serono, Genentech/Roche | Novantrone, Ocrevus |
| PIPE-307 | Relapse remitting multiple sclerosis | N/A | Over 20 DMTs not promoting remyelination |
| PIPE-307 | Depression | N/A | SSRIs, SNRIs, Antipsychotics, Mood stabilizers, Generics |
| CTX-343 | Undisclosed | N/A | N/A |
Weekly analyses of biotech startups, generated by AI
Receive high quality, AI-generated analyses of biotech startups, public companies and scientific papers each week.
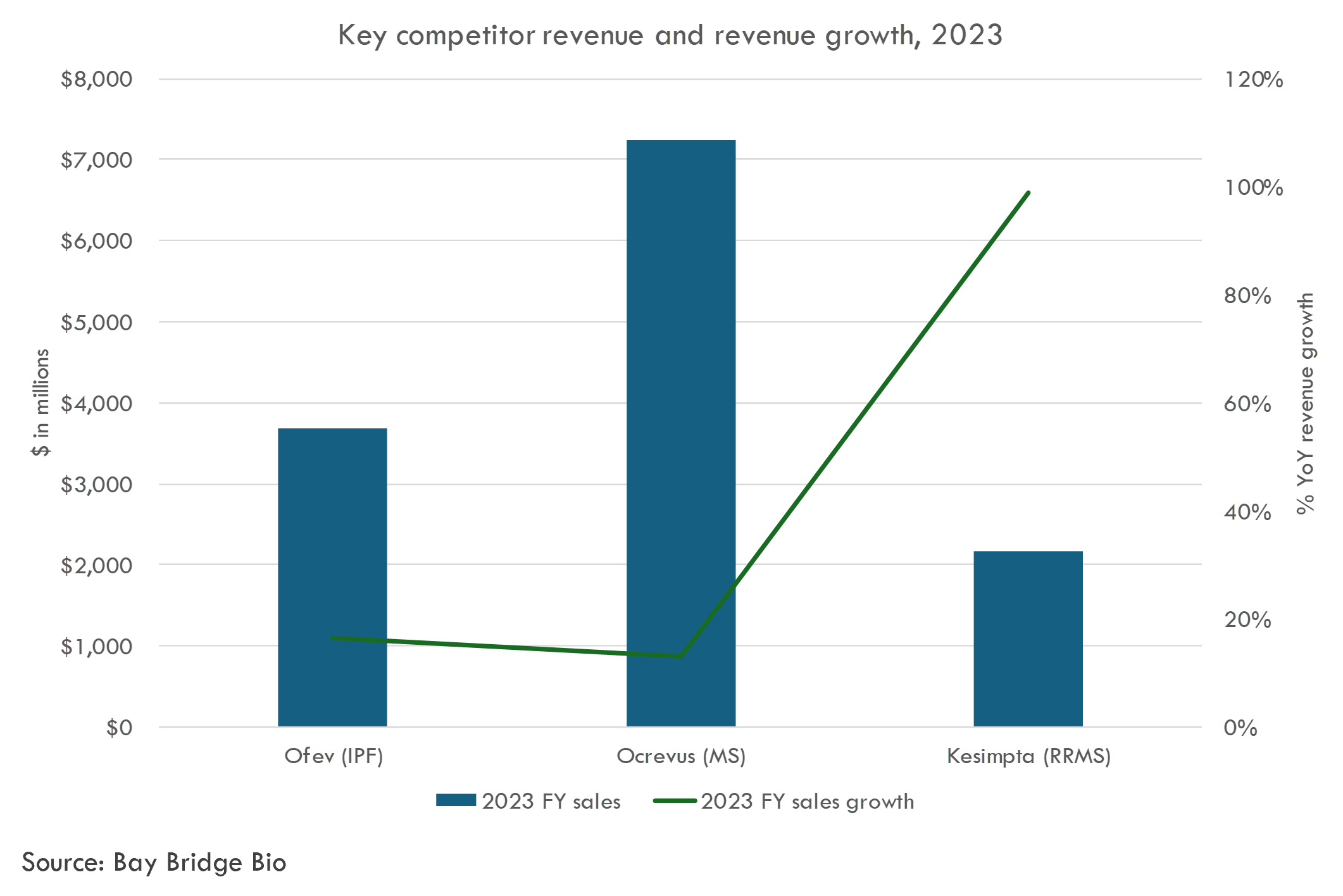
Several of these competitors generate several billion in revenue each year, illustrating the market potential in Contineum's target indications.
Market overview
Idiopathic pulmonary fibrosis
Idiopathic Pulmonary Fibrosis (IPF) is a chronic and progressive disease that affects the lungs. It is characterized by the thickening and scarring (fibrosis) of the lung tissue, which is idiopathic, meaning its cause is unknown. This scarring process is believed to result from a complex interaction of genetic, environmental, and internal factors, but the exact mechanism remains elusive.
Pathology:
The hallmark of IPF is the pattern of fibrosis seen in the lung tissue, known as usual interstitial pneumonia (UIP). UIP is characterized by the presence of fibroblastic foci, areas where the lung tissue shows active fibrosis, amidst areas of normal lung and dense scar tissue. Over time, the lung tissue becomes progressively scarred, leading to a decrease in lung function. The disease primarily involves the peripheral and lower portions of the lungs.
Symptoms:
Common symptoms of IPF include chronic, progressive shortness of breath (dyspnea), especially during physical activity, and a dry, hacking cough. Other symptoms may include fatigue, weakness, discomfort in the chest, loss of appetite, and unexplained weight loss. As the disease progresses, patients may develop clubbing of the fingers and toes (due to changes in the amount of soft tissue beneath the nail beds) and cyanosis (bluish discoloration of the skin and mucous membranes due to low oxygen levels).
Prognosis:
IPF is a serious condition with a variable prognosis. The median survival time after diagnosis is typically 3 to 5 years, but disease progression varies widely among individuals. Some patients experience rapid deterioration, while others may have periods of stability. Factors that can influence prognosis include age, overall health, extent of lung function impairment at diagnosis, and response to treatment.
Treatment:
There is no cure for IPF, and treatment aims to slow the progression of the disease, alleviate symptoms, and improve quality of life. Antifibrotic medications, such as pirfenidone and nintedanib, have been shown to slow disease progression. Supportive treatments include oxygen therapy to help with breathing, pulmonary rehabilitation to improve physical functioning and alleviate symptoms, and lifestyle changes, including smoking cessation and regular, gentle exercise. Lung transplantation may be considered for suitable candidates.
Given the complex nature of IPF, its treatment and management typically involve a multidisciplinary approach that includes pulmonologists, radiologists, pathologists, and sometimes rheumatologists, along with specialized nurses and respiratory therapists. Research into the underlying mechanisms of IPF and the development of new therapeutic strategies continues to be a critical area of scientific inquiry, holding hope for better outcomes in the future.
PIPE-791 comes into the IPF (Idiopathic Pulmonary Fibrosis) market landscape with a compelling profile, presenting itself as a novel, high affinity, brain penetrant, small molecule LPA1R (lysophosphatidic acid receptor 1) antagonist. Its unique features, which include high bioavailability, low plasma protein binding, and long receptor residence time based on preclinical studies, suggest it has the potential to be a differentiated therapeutic option in the management of IPF.
Market Context and Unmet Need
The current standard of care in IPF consists of two FDA-approved therapies: pirfenidone (Esbriet) and nintedanib (Ofev). Both medications have been proven to slow the progression of the disease; however, they do not halt it entirely. Additionally, their use is often limited by side effects, tolerability issues, and the requirement for multi-daily dosing regimens. Given the progressive nature of IPF, which leads to severe respiratory function loss and has a prognosis worse than many cancers—where approximately 60% to 80% of patients succumb to respiratory failure within five years of diagnosis—the market is acutely in need of novel treatments. With an estimated 130,000 patients in the United States and three million cases worldwide as of 2017, the demand for more effective and better-tolerated therapies is substantial.
Opportunity for PIPE-791
The development of PIPE-791 is particularly exciting given the pathological role of the LPA/LPA1R pathway in IPF. The pathway's activation, through elevation of LPA in response to lung injury, is a critical mediator of fibrosis. It drives a number of cellular processes, such as fibroblast recruitment and vascular leakage, that culminate in fibrosis. Therefore, an antagonist like PIPE-791, with a focus on inhibiting LPA1, has the potential to directly target and modify the disease's course by reducing fibrosis.
The preclinical and Phase 1 data supporting the continued development of PIPE-791 for IPF highlight its distinctiveness versus other LPA1R antagonists currently in development. Its high bioavailability and favorable pharmacokinetic profile, in tandem with the ongoing clinical investigation including the planned Phase 1b open-label trial to measure lung and brain receptor occupancy, shine a promising light on its potential efficacy and patient compliance.
Moreover, IPF’s status as a condition with high unmet medical need underlines the significant market opportunity for PIPE-791. If clinical trials confirm its safety profile and efficacy in modulating the fibrotic processes underlying IPF, PIPE-791 could not only establish itself as a leading treatment option but also substantially impact patient outcomes in a positive way. Given its novel mechanism of action and the preliminary data indicating a potentially superior profile, PIPE-791 could capture a significant share of the IPF market, providing a much-needed alternative to the current standard of care.
Lastly, the broader exploration of PIPE-791's utility in disorders where the LPA1 pathway is implicated, alongside its IPF application, could further validate the versatile potential of this compound, expanding its market reach and fulfilling deeply felt unmet needs across multiple indications.
In the evolving landscape of Idiopathic Pulmonary Fibrosis (IPF) treatment, several promising therapies are under development. These experimental treatments, like PIPE-791, aim to address the significant unmet medical needs within the IPF community by targeting various pathways implicated in the disease's pathogenesis. Here, we'll discuss some of the notable competitors that PIPE-791 may face, based on both their mechanisms of action and the stage of clinical development.
| Company Name | Product Name | Latest Clinical Phase | Target | Time Period of Measurement | Relative Reduction in the Rate of Change in ppFVC Versus Placebo | Key Clinical Studies |
|---|---|---|---|---|---|---|
| Genentech/Roche | Esbriet | Approved | TGF-beta, PDGF pathways | 1 year | ~50% | ASCEND, CAPACITY |
| Boehringer Ingelheim | Ofev | Approved | FGFR, PDGFR, VEGFR | 1 year | ~50% | INPULSIS-1, INPULSIS-2 |
| Boehringer Ingelheim | BI 1015550 | Phase 3 | PDE4B | 12 weeks | Superior to placebo with a median difference of 88.4 ml (without background antifibrotic use) and 62.4 ml (with background antifibrotic use) | Phase 2 (NCT04419506) |
| Bristol Myers Squibb | BMS-986278 | Phase 2 | LPA1 | 26 weeks | 62% | Phase 2 (name not provided in press release) |
| AbbVie | DJS-002 | Phase 1 | Unknown | Data not available | Data not specified | Early stage, no named studies |
| Horizon/Amgen | Fipaxalparant / HZN-825 | Phase 2 | LPA1 | Data not available | Data not specified | Phase 2 ongoing |
| Structure Therapeutics | LTSE-2578 | Preclinical | Unknown | Data not available | Data not specified | Preclinical, no studies |
| United Therapeutics | treprostinil | Approved (for PAH) | Prostacyclin (IP) receptor | Data not available | Data not specified | TRIUMPH I (for PAH) |
| Pliant Therapeutics | Bexotegrast | Phase 2a | Integrin αvβ6 | 12 weeks | Statistically significant mean increase of +140 mL in FVC from baseline at 12 weeks compared to placebo | INTEGRIS-IPF (Phase 2a) |
| Redx Pharma | zelasudil (RXC007) | Phase 1 | Rho-associated protein kinase (ROCK) | Data not available | Data not specified | Early stage, no named studies |
| Redx Pharma | RXC006/AXD5055 | Preclinical | Unknown | Data not available | Data not specified | Preclinical, no studies |
| Endeavor Biomedicines | ENV-101 | Phase IIa | Hedgehog (Hh) signaling pathway | 12 weeks + 6 weeks observation | Data not available | Phase IIa trial in IPF patients |
There are two mainstays in the pharmacological treatment of Idiopathic Pulmonary Fibrosis (IPF) that have significantly impacted the management of the disease. Both drugs have been pivotal in providing options beyond supportive care, which was the limit before their availability.
- Nintedanib (Ofev): Approved by the FDA in 2014, Nintedanib is a small molecule tyrosine kinase inhibitor that targets multiple pathways involved in fibrosis, including VEGF, FGF, and PDGF receptors. Its broad mechanism of action allows it to tackle the fibrotic process from various angles. Clinical trials have demonstrated that Nintedanib can slow the decline of lung function in IPF patients, measured by Forced Vital Capacity (FVC), thereby altering the course of the disease's progression. Its side effects include diarrhea, nausea, abdominal pain, and liver enzyme elevations, which require monitoring.
- Pirfenidone (Esbriet): Also approved in 2014, Pirfenidone is an anti-fibrotic and anti-inflammatory drug. While its exact mechanism of action is not fully understood, it is believed to inhibit the synthesis of TGF-beta, a key molecule involved in fibrogenesis and inflammatory processes. Like Nintedanib, Pirfenidone has been shown to slow the rate of FVC decline in patients with IPF, offering another therapeutic option for managing the disease. The common side effects of Pirfenidone include gastrointestinal discomfort, rash, and photosensitivity.
Investigational Drugs:
Several investigational drugs are in various stages of development, targeting different pathways implicated in the pathogenesis of IPF. These include:
- Antifibrotic agents that aim to directly inhibit the process of fibrosis.
- Anti-inflammatory drugs intended to reduce the inflammatory responses that contribute to fibrotic changes.
- Tyrosine kinase inhibitors, similar to Nintedanib but perhaps with different targets or better efficacy/side effect profiles.
- Biologic therapies, including monoclonal antibodies targeting specific molecules or pathways involved in fibrosis.
The hope is that these investigational drugs, upon successful development and approval, will offer more options for IPF treatment, perhaps with better efficacy, improved safety profiles, or easier dosing regimens. This is crucial in a disease like IPF, where the existing treatments can slow but not stop disease progression, and significant variability exists in patient responses and tolerability to treatment.
Current Standard of Care for IPF
The standard of care for IPF currently includes two FDA-approved drugs, Nintedanib (Ofev) and Pirfenidone (Esbriet), which have been shown to slow the progression of the disease but do not stop it. Both drugs can have side effects that may affect patient compliance and quality of life. Therefore, there remains a significant unmet need for novel treatments that can offer better efficacy, fewer side effects, or both.
Potential Impact of PIPE-791
PIPE-791, as a novel, high affinity, brain-penetrant small molecule LPA1R antagonist, targets a distinct pathway related to fibrosis. Given the role of LPA/LPA1R signaling in fibrosis, inhibition through PIPE-791 could offer a new mechanism to prevent or slow down the fibrotic process in IPF. Here's how PIPE-791 might fit into the current treatment paradigm:
- Novel Mechanism of Action: PIPE-791’s targeting of the LPA1R pathway represents a different approach compared to the mechanisms of Nintedanib and Pirfenidone. This could offer an alternative for patients who do not respond adequately to current therapies or for whom side effects are intolerable.
- Potential Combination Therapy: Considering IPF's complex pathogenesis, combining drugs with different mechanisms could potentially provide a more effective treatment strategy than monotherapy. If PIPE-791 is shown to be safe and effective, it might be used in combination with one of the current standard treatments to provide a synergistic effect.
- Safety and Tolerability: The success of PIPE-791 will also depend on its safety and tolerability profile. If it demonstrates fewer or more manageable side effects compared to existing therapies, it could become a preferred treatment option, assuming its efficacy is at least comparable.
- Quality of Life Considerations: Beyond efficacy and safety, PIPE-791’s impact on the quality of life will be an important factor. If the drug can offer less frequent dosing, better tolerability, or improved lung function beyond the slowing of disease progression, it could significantly benefit IPF patients.
- Post-Market and Real-World Data: After potential approval, the real-world efficacy and patient adherence to PIPE-791 will be critical in determining its place in the standard of care. Real-world data will provide further insights into how PIPE-791 performs outside clinical trial settings, which can influence prescribing practices.
In summary, PIPE-791 has the potential to significantly impact the treatment landscape of IPF, provided ongoing and future clinical trials confirm its efficacy and safety. Its success will depend not only on its clinical performance relative to existing therapies but also on factors such as patient preference, side effect profile, and potential for use in combination therapies. Given the unmet needs in IPF treatment, novel therapies like PIPE-791 are eagerly anticipated by both clinicians and patients.
Preclinical data
The preclinical data for PIPE-791, a novel, high-affinity, brain-penetrant, small molecule LPA1R antagonist, presents a promising profile for its development in treating idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis. The summary of the studies and key results is as follows:
-
Toxicology Studies:
-
Rodent and Minipig Toxicology: PIPE-791 was evaluated through oral dosing in Sprague Dawley rodents and Göttingen minipigs across single-dose, 14-day, and 28-day Good Laboratory Practice (GLP) toxicology studies. For both species, a no-observed-adverse-effect level (NOAEL) was established at 1000 mg/kg/day, the highest dose tested, with no adverse effects or findings of toxicological significance observed at any dose level.
-
Genotoxicity: PIPE-791 was found to be negative for mutagenicity and clastogenicity in both in vitro (Ames test, chromosomal aberrations assay) and in vivo (rodent bone marrow) studies, supporting its genetic safety profile.
-
Based on these results, chronic toxicity studies have been initiated, extending over six months for rodents and nine months for minipigs, starting January 2024.
-
-
Preclinical Proof-of-Concept Studies:
-
In Vitro Studies:
-
LPA1R Antagonism: PIPE-791 exhibited single-digit nanomolar potency against human LPA1R in a competitive membrane filter binding assay. It showed slow association and dissociation kinetics, with increased potency observed after longer pre-incubation in a calcium mobilization assay, indicative of its slow on-rate kinetics.
-
Selectivity: The molecule displayed over 30-fold selectivity against LPA2 and LPA3 receptor isoforms and showed no appreciable activity against 78 other targets tested at 30 µM, underscoring its specificity.
-
-
In Vitro Functional Assays:
-
Fibroblast Chemotaxis and Collagen Production: PIPE-791 effectively inhibited LPA-induced chemotaxis in primary human fibroblasts and LPA-induced collagen production in lung fibroblasts, with IC50 values of 1.5 nM and 1.14 nM, respectively. This suggests its potential in mitigating fibrosis by interfering with fibroblast activity.
-
-
In Vivo Studies:
-
LPA1R Occupancy: Using a selective radioligand, oral dosing of PIPE-791 demonstrated dose-dependent inhibition of LPA1R, aligning with its in vitro binding affinity and indicating effective in vivo receptor occupancy at low doses.
-
Histamine Response Inhibition: PIPE-791 inhibited LPA-induced plasma histamine release after steady-state dosing, with an ED50 of approximately 0.03 mg/kg, suggesting effective pharmacodynamic activity at low plasma concentrations.
-
Lung Fibrosis Model: In a rodent model of IPF, treatment with PIPE-791 post-bleomycin instillation led to increased survival, dose-dependent reduction in lung tissue fibrosis, and improved body weights, demonstrating its therapeutic potential against lung fibrosis.
-
-
The comprehensive preclinical evaluation of PIPE-791 highlights its potential as a safe and effective treatment for IPF and possibly progressive multiple sclerosis, with favorable toxicity, specificity, and efficacy profiles. The ongoing long-term toxicity studies will further elucidate its safety profile, supporting its progression into clinical development.
The preclinical findings for PIPE-791 are significant for several reasons, providing a foundational understanding of its potential efficacy and safety profile as a novel treatment for idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis. The data suggests a robust safety margin, demonstrated by the lack of adverse effects or toxicological findings in both rodent and minipig models up to the highest dose tested. Additionally, the lack of genotoxicity supports the compound's safety profile, an essential aspect as it progresses through the development pipeline.
Predictive Validity and Translatability of Disease Models:
-
Rodent Models for IPF: The use of a bleomycin-induced lung fibrosis model in rodents is a standard approach to study the efficacy of antifibrotic compounds. While this model mimics certain aspects of human IPF, including the development of fibrosis following lung injury, it does not fully recapitulate the complex, progressive nature of human IPF. Rodent models can overestimate the efficacy of therapeutic agents due to differences in disease etiology, immune responses, and fibrotic processes between rodents and humans. Despite these limitations, the dose-dependent reduction in lung fibrosis and improved survival rates observed with PIPE-791 provide valuable evidence of its potential therapeutic effects. The translatability of these findings to humans will depend on further validation in more complex models and ultimately, clinical trials.
-
In Vitro Assays and Receptor Occupancy Studies: The high affinity of PIPE-791 for the LPA1 receptor and its selectivity demonstrated in vitro are promising for its specificity and potential efficacy. However, translating these findings to clinical efficacy requires careful consideration. Receptor occupancy and functional assays in animal models offer insights into the drug's mechanism of action and potential pharmacodynamic effects in humans. The predictive validity of these models largely depends on the similarity of the LPA1R signaling pathways and fibrotic processes between the model systems and humans.
Differences in Model Systems and Human Biology:
-
Species-Specific Responses: Differences in drug metabolism, immune system function, and pathophysiology between rodents, minipigs, and humans can influence the translatability of preclinical findings. For example, the expression and regulation of LPA receptors and the fibrotic response to injury may vary, potentially affecting the drug's efficacy and safety profile in humans.
-
Genotoxicity and Safety Profiles: The negative results in genotoxicity assays are reassuring but must be interpreted with caution. The predictive validity of these tests for human outcomes, while generally good, is not absolute. Long-term clinical safety cannot be fully assured by preclinical studies alone due to differences in cellular responses and DNA repair mechanisms between species.
-
Chronic Toxicity Studies: The initiation of longer-term toxicity studies in rodents and minipigs is a critical step towards addressing the potential for cumulative or delayed adverse effects not captured in short-term studies. These studies are more reflective of chronic human exposure and will provide important data on the safety profile of PIPE-791 over extended periods.
In conclusion, while the preclinical studies of PIPE-791 are promising and indicate potential therapeutic benefits for IPF and progressive multiple sclerosis, the translation of these findings to clinical success requires careful navigation of the differences between model systems and human biology. Subsequent phases of clinical research will be crucial for validating these preclinical results and determining the safety and efficacy of PIPE-791 in humans.
The preclinical and Phase 1 data for PIPE-791, in comparison with other LPA1R antagonists, position it as a potentially differentiated therapy for idiopathic pulmonary fibrosis (IPF) and progressive multiple sclerosis (MS). Key factors that could contribute to PIPE-791's clinical differentiation include its high bioavailability, low plasma protein binding, long receptor residence time, and a strategic design to mitigate hepatobiliary toxicity.
Clinical Significance of Findings:
-
Hepatobiliary Toxicity Mitigation: A notable differentiation point for PIPE-791 is its minimal interaction with bile salt export pump (BSEP), a mechanism implicated in the hepatobiliary toxicity observed with first-generation LPA1R antagonist BMS-986020. BMS-986020 showed significant hepatotoxicity in preclinical studies, which was a major safety concern. PIPE-791’s design specifically addresses this issue, presenting a lower risk for similar toxicity at its clinically efficacious dose, which is anticipated to be under 10 mg QD. This could significantly enhance its safety profile, making it a more viable option for long-term treatment of chronic diseases like IPF and progressive MS.
-
24-Hour Receptor Coverage: Unlike other LPA1R antagonists, PIPE-791 demonstrates the ability to maintain plasma concentrations above the functional IC50 for LPA1R over 24 hours with a single oral dose. This extended receptor coverage suggests that PIPE-791 could offer sustained efficacy with once-daily dosing, improving patient compliance and therapeutic outcomes.
Potential for Meaningful Clinical Differentiation:
-
Safety and Efficacy Balance: The avoidance of BSEP inhibition and the resultant low risk of hepatobiliary toxicity, combined with high receptor occupancy, positions PIPE-791 as a potentially safer and more efficacious option compared to existing LPA1R antagonists. This balance is crucial for chronic conditions like IPF and MS, where long-term treatment is necessary.
-
Pharmacokinetic Advantages: The high oral bioavailability and metabolic stability of PIPE-791, coupled with its low plasma protein binding, ensure that a greater fraction of the administered dose remains available to exert its therapeutic effect. These pharmacokinetic properties are essential for achieving consistent drug exposure and therapeutic effects across the dosing interval.
-
Clinical Development and Patient Compliance: The features of PIPE-791 not only suggest a favorable safety and efficacy profile but also imply potential advantages in clinical development and patient compliance. A lower dose, once-daily regimen can simplify treatment schedules, reduce the burden of adverse effects, and potentially lead to better patient outcomes.
The comparative preclinical data for PIPE-791 suggests it has a significant potential for clinical differentiation from other LPA1R antagonists currently in development for IPF and progressive MS. Its design and pharmacological profile address some of the key limitations observed with first-generation LPA1R antagonists, particularly concerning safety and dosing convenience. These attributes may translate into meaningful clinical benefits, highlighting PIPE-791 as a promising candidate in the landscape of LPA1R-targeted therapies. Further clinical trials will be essential to validate these preclinical findings and fully assess the therapeutic potential of PIPE-791 in human populations.
Progressive Multiple Sclerosis
Progressive Multiple Sclerosis (MS) represents a form of Multiple Sclerosis that continually worsens over time without distinct relapses (exacerbations) or periods of remission. Unlike Relapsing-Remitting MS (RRMS), which is characterized by clear episodes of neurological dysfunction followed by periods of partial or complete recovery, Progressive MS is marked by a steady accumulation of disability. It can be further classified into Primary Progressive MS (PPMS), where the disease is progressive from the onset, and Secondary Progressive MS (SPMS), where the disease transitions to a progressive course after an initial relapsing-remitting phase.
Pathology:
The underlying pathology of Progressive MS involves inflammation, demyelination, and neurodegeneration within the central nervous system (CNS). In Progressive MS, there is a continuous loss of neurons and brain atrophy, which is more pronounced compared to RRMS. The pathological hallmarks include demyelinated plaques in the brain and spinal cord, axonal loss, and gliosis. The mechanisms contributing to progression are complex and involve chronic inflammation, microglial activation, mitochondrial dysfunction, and iron accumulation, leading to an environment that is not conducive to remyelination and neuronal repair.
Symptoms:
The symptoms of Progressive MS are diverse and depend on the areas of the CNS that are affected. Common symptoms include:
- Muscle weakness and spasticity
- Difficulty walking and coordination problems (ataxia)
- Fatigue, one of the most disabling symptoms
- Cognitive changes, including problems with memory and concentration
- Bladder and bowel dysfunction
- Visual disturbances
- Pain and sensory changes
Due to the progressive nature of this condition, symptoms typically worsen over time, leading to increased disability.
Prognosis:
The prognosis of Progressive MS can vary significantly among individuals. PPMS tends to progress more steadily without remission, while the course of SPMS can vary, with some individuals experiencing occasional plateaus or minor improvements. Overall, individuals with Progressive MS often experience a gradual increase in disability, which can lead to mobility issues and require the use of assistive devices. The rate of progression and the severity of symptoms vary widely, influenced by factors such as age at onset, initial symptoms, and the extent of neurodegeneration.
Treatment:
Treatment options for Progressive MS are more limited compared to RRMS, and managing the disease focuses on slowing progression, managing symptoms, and improving quality of life. For PPMS, the FDA-approved treatment options include ocrelizumab (Ocrevus), which has shown to slow disease progression. For SPMS, options like siponimod (Mayzent), ocrelizumab, and mitoxantrone are available, aiming at modifying the disease course. Symptomatic treatments address specific symptoms such as muscle spasticity, bladder dysfunction, and fatigue. Rehabilitation therapies, including physical and occupational therapy, are crucial for maintaining function and mobility.
Research into Progressive MS is ongoing, with a focus on understanding the mechanisms driving disease progression and developing novel therapeutic strategies that can promote remyelination, neuroprotection, and repair. The unmet need for effective treatments in Progressive MS remains significant, underscoring the importance of continued research and innovation in this field.
The development of PIPE-791 for Progressive Multiple Sclerosis (MS) represents a strategic move into a therapeutic area characterized by significant unmet needs and a growing understanding of the disease's underlying biological mechanisms. Progressive MS, encompassing both Primary Progressive MS (PPMS) and Secondary Progressive MS (SPMS), presents a landscape where few effective treatment options are currently available.
Market Context and Unmet Need:
Progressive MS affects a portion of the MS population that has historically been underserved by clinical advancements. The primary forms of MS treatments have been focused on Relapsing-Remitting MS (RRMS), with disease-modifying therapies (DMTs) designed to reduce relapses and new lesion formation. For Progressive MS, especially PPMS, treatment options have been limited, reflecting a significant gap in addressing the steady progression of disability seen in these patients.
Ocrelizumab (Ocrevus) by Genentech/Roche is the first and only medication approved for both RRMS and PPMS, demonstrating the scarcity of options tailored to the progressive form of the disease. Siponimod (Mayzent) by Novartis has received FDA approval for SPMS with active disease, characterized by relapses or imaging features of inflammatory activity.
Below is a table highlighting approved MS drugs:
| Drug Name | Approved Indications | Phase 3 Studies | Annualized Relapse Rate (ARR) | Placebo Arm's ARR | Active Control | % Disability Progression at 12 Weeks |
|---|---|---|---|---|---|---|
| Ocrevus | Primary Progressive MS (PPMS), Relapsing-Remitting MS (RRMS), Active Secondary Progressive MS (SPMS) | OPERA I and II, ORATORIO | Not applicable for PPMS; ~0.16 for RRMS (OPERA studies) | Not applicable for PPMS; ~0.32 for RRMS (OPERA studies) | Interferon beta-1a ARR: ~0.29 (OPERA) | 32.9% with Ocrevus vs. 39.3% with placebo (ORATORIO) |
| Tecfidera | Relapsing forms of MS, including RRMS, SPMS with active disease, Clinically Isolated Syndrome (CIS) | DEFINE, CONFIRM | ~0.17 (DEFINE twice daily); ~0.19 (DEFINE thrice daily); ~0.22 (CONFIRM twice daily); ~0.20 (CONFIRM thrice daily) | ~0.36 (DEFINE); ~0.40 (CONFIRM) | Glatiramer acetate ARR: ~0.29 (CONFIRM) | None |
| Gilenya | Relapsing forms of MS, including RRMS and CIS | FREEDOMS, FREEDOMS II, TRANSFORMS | ~0.18 (FREEDOMS); ~0.21 (FREEDOMS II, 0.5 mg); ~0.16 (TRANSFORMS) | ~0.40 (FREEDOMS); ~0.40 (FREEDOMS II); ~0.40 (TRANSFORMS control group) | None | None |
| Tysabri | Relapsing forms of MS, including RRMS, SPMS with active disease | AFFIRM, SENTINEL | ~0.24 (AFFIRM); Combined with placebo in SENTINEL | ~0.74 (AFFIRM); Combined with another drug in SENTINEL | None | None |
| Kesimpta | Relapsing forms of MS, including RRMS, CIS, and active SPMS | ASCLEPIOS I and II | ~0.11 (ASCLEPIOS I and II) | ~0.35 (ASCLEPIOS I and II) | None | None |
| Novantrone | Secondary Progressive MS (SPMS), worsening RRMS, progressive-relapsing MS | IMPACT, MITOX | Not primarily focused on ARR | Not primarily focused on ARR | None | None |
| Aubagio | Relapsing forms of MS, including RRMS and SPMS with active disease | TEMSO, TOWER, TOPIC | ~0.32 (TEMSO); ~0.36 (TOWER) | ~0.54 (TEMSO); ~0.50 (TOWER) | None | None |
| Ponvory | Relapsing forms of MS, including RRMS, CIS, and active SPMS | OPTIMUM | ~0.20 (OPTIMUM) | ~0.29 (OPTIMUM) | None | None |
| Mavenclad | Relapsing forms of MS, including RRMS, SPMS with active disease, and CIS | CLARITY, ORACLE-MS, CLARITY Extension | ~0.14 (CLARITY); ~0.15 (CLARITY Extension) | ~0.33 (CLARITY); Not specified for CLARITY Extension | None | None |
Potential of PIPE-791:
- Novel Target: PIPE-791's targeting of the LPA1R pathway could influence neuroinflammation and neurodegeneration, both key components of progressive disease pathology. By potentially addressing the underlying mechanisms of progression, PIPE-791 could fill a gap not currently met by existing treatments.
- Clinical Validation: The clinical validation of LPA1R antagonism in other indications, coupled with the high bioavailability, low plasma protein binding, and long receptor residence time of PIPE-791, suggests a favorable profile for therapeutic development.
- High Unmet Medical Need: The limited options for Progressive MS underscore a high unmet medical need. A successful introduction of PIPE-791 could not only benefit patients by slowing disease progression but also capture a significant market share due to the scarcity of direct competitors.
- Competitive Advantage: The potential for PIPE-791 to offer a differentiated mechanism of action, alongside favorable pharmacokinetic properties, positions it advantageously against the backdrop of existing therapies like Ocrevus and Mayzent. Its oral administration could offer convenience over infusion-based therapies, aligning with patient preference for less invasive treatment modalities.
Conclusion:
The development and potential approval of PIPE-791 for Progressive MS could represent a significant advance in the treatment landscape, offering hope to patients with few therapeutic options. Given the complexity of Progressive MS and the varying patient needs, PIPE-791 could become an integral part of a multifaceted treatment approach, either as a standalone therapy or in combination with other modalities aimed at managing specific symptoms or aspects of the disease.
The success of PIPE-791 will ultimately depend on the outcomes of clinical trials, specifically demonstrating efficacy in slowing disease progression and an acceptable safety profile. With robust clinical data, PIPE-791 could address a critical unmet need, benefiting patients and establishing a strong market presence in the treatment of Progressive MS.
The landscape for Progressive Multiple Sclerosis (MS) treatment is evolving, with several promising therapies under development. These novel treatments target various aspects of Progressive MS pathology, aiming to slow disease progression, reduce symptoms, and improve quality of life for patients. Given the unique mechanism of PIPE-791 as an LPA1R antagonist, it's essential to consider the variety of other mechanisms being explored in the pipeline that could serve as potential competition. Here are some notable examples of treatments in development that might compete with PIPE-791:
- BTK Inhibitors:
- Bruton's tyrosine kinase (BTK) inhibitors are a class of drugs being investigated for their role in modulating the immune system and potentially protecting against neurodegeneration. Candidates like EVICTION (fenebrutinib) and tolbrutinib are under investigation for both Relapsing-Remitting and Progressive MS forms. These drugs could provide broad therapeutic benefits across the MS spectrum, including Progressive MS.
- Siponimod (Mayzent):
- Currently approved for Secondary Progressive MS (SPMS) with active disease, Siponimod represents the expansion of treatment options for progressive forms of MS. It's an S1P receptor modulator that works by trapping immune cells in lymph nodes, preventing them from entering the CNS and causing damage. Ongoing research and expanded indications could further cement its role in Progressive MS treatment paradigms.
- Ocrelizumab (Ocrevus):
- This CD20-directed cytolytic antibody is the first drug approved for Primary Progressive MS (PPMS) and has significantly impacted the treatment landscape. Its efficacy in reducing disease progression in PPMS makes it a direct competitor for any new treatments targeting this MS form.
- Stem Cell Therapies:
- Hematopoietic stem cell transplantation (HSCT) aims to reset the immune system to stop it from attacking the central nervous system. Although primarily explored in Relapsing-Remitting MS, ongoing research into optimizing protocols could extend its application into Progressive MS, offering a potentially curative approach.
- CAR-T Cell Therapies:
- CD19 CAR-T cell therapies are designed to treat autoimmune disease by depleting B-cells. These therapies have shown durable responses in small clinical studies in other autoimmune diseases, and are entering the clinic in multiple sclerosis.
- Neuroprotective and remyelination strategies:
- Drugs that promote remyelination or protect neurons from degeneration represent a novel approach to treating MS. Agents such as Clemastine, traditionally an antihistamine, and high-dose biotin are being explored for their potential neuroprotective and remyelinating capabilities in Progressive MS. Additionally, molecules targeting the repair pathway, such as opicinumab (anti-LINGO-1 antibody), though facing setbacks in clinical trials, highlight ongoing research in this direction.
- Masitinib:
- An oral tyrosine kinase inhibitor targeting mast cells and microglia, masitinib is under investigation for Progressive MS. By modulating these inflammatory cells, it has the potential to reduce neuroinflammation and neurodegeneration associated with Progressive MS.
The competitive landscape for Progressive MS treatments is characterized by a diversity of mechanisms, reflecting the complex pathology of the disease. The success of PIPE-791 will depend not only on its efficacy and safety profile but also on how it compares to these emerging therapies, both existing and in development. Given the high unmet medical need in Progressive MS, there is significant room for multiple therapeutic approaches that can offer benefits over the current standard of care, either as standalone treatments or in combination regimens. PIPE-791’s unique mechanism through LPA1R antagonism presents an innovative approach; however, its ultimate position in the treatment algorithm will be determined by clinical trial outcomes and its ability to offer differentiated benefits in terms of efficacy, safety, and patient quality of life.
As of early 2023, the treatment landscape for Progressive Multiple Sclerosis (MS), which includes both Primary Progressive MS (PPMS) and Secondary Progressive MS (SPMS), has seen some notable advancements, although the options remain significantly fewer compared to Relapsing-Remitting MS (RRMS). Here are some of the key therapies:
Ocrelizumab (Ocrevus)
Approved by the FDA in 2017, Ocrelizumab was the first drug to gain approval for the treatment of PPMS, marking a milestone in Progressive MS treatment. Ocrelizumab is a humanized monoclonal antibody designed to target CD20-positive B cells, which are believed to play a key role in the pathogenesis of MS by contributing to myelin and neuronal damage. For patients with PPMS, Ocrelizumab has shown to modestly slow the progression of disability. It's also approved for RRMS, making it a versatile option across different MS types.
Siponimod (Mayzent)
Siponimod, approved by the FDA in 2019, is indicated for adults with SPMS who are experiencing progression of disease. It's a sphingosine 1-phosphate (S1P) receptor modulator, working by trapping immune cells in lymph nodes to reduce their ability to contribute to CNS damage. Clinical trials have demonstrated that Siponimod can slow the progression of disability in SPMS and reduce the rate of brain volume loss, addressing both inflammatory and neurodegenerative components of MS.
Cladribine (Mavenclad)
Though primarily approved for RRMS, Cladribine has shown potential in treating SPMS with active disease (characterized by relapses or evidence of new MRI activity). It works by selectively reducing certain types of white blood cells, particularly lymphocytes, that are implicated in the MS inflammatory process. By doing so, it aims to lower the frequency of relapses and slow down the progression of the disease.
Recently Approved Drugs:
As of the last knowledge update in there weren't new specific approvals for Progressive MS beyond those mentioned. However, the landscape of treatment options is constantly evolving with ongoing clinical trials aimed at addressing the significant unmet needs in Progressive MS treatment.
The treatment landscape for Progressive MS has appreciated meaningful advancements with the introduction of drugs like Ocrelizumab and Siponimod, specifically designed to address the progressive forms of MS. However, given the complex nature of Progressive MS and the variability of its progression among patients, there remains a pressing need for additional effective treatments. Future developments hinge on ongoing research into the underlying mechanisms of disease progression and the translation of this knowledge into novel therapeutic strategies.
Given the current landscape of treatment options specifically targeting Progressive Multiple Sclerosis (MS), including both Primary Progressive MS (PPMS) and Secondary Progressive MS (SPMS), PIPE-791 represents an intriguing new potential addition. Its unique mechanism of action, targeting the LPA1R pathway, differentiates it from the existing treatments and offers a novel approach that could complement or enhance the current standard of care.
Current Standard of Care for Progressive MS:
For PPMS, Ocrelizumab (Ocrevus) is a pivotal treatment, being the first and only therapy approved specifically for this form of MS. In the case of SPMS, treatment options include Siponimod (Mayzent), which has been shown to be beneficial for patients with active disease (evidenced by relapses or MRI activity). Other treatments, such as Cladribine (Mavenclad), while not specifically approved for SPMS, may also be considered for patients with active disease characteristics.
Potential Role of PIPE-791:
- Novel Mechanism of Action: PIPE-791, with its distinct pharmacological target in the LPA1R pathway, introduces a new therapeutic direction. The LPA1R pathway has been implicated in various pathological processes, including neuroinflammation and fibrosis, which are relevant to the progression of MS. By inhibiting this pathway, PIPE-791 may offer neuroprotective effects or hinder disease progression through mechanisms not directly targeted by current therapies.
- Combination Therapy Possibility: Given the multifactorial nature of Progressive MS, combining therapies with complementary mechanisms of action could be an effective strategy. PIPE-791 might be used in tandem with approved therapies like Ocrelizumab or Siponimod, potentially offering additive or synergistic effects, particularly in slowing disease progression or ameliorating symptoms associated with Progressive MS.
- Addressing Unmet Needs: Progressive MS remains a challenging form of MS to treat effectively, with a substantial unmet medical need for therapies that can more significantly slow or halt disease progression and improve patient quality of life. If clinical trials show that PIPE-791 is effective in these areas, it could become an essential part of the treatment regimen for Progressive MS, either as a monotherapy or in combination with other drugs.
- Safety and Tolerability Profile: The ultimate role of PIPE-791 in the treatment landscape will also depend on its safety and tolerability profile compared to existing treatments. A favorable side effect profile and ease of administration (for example, oral administration versus infusion) could make PIPE-791 a preferred option for some patients.
As the drug development process for PIPE-791 continues, with Phase 1b trials planned to inform dose selection for future studies in Progressive MS, there is cautious optimism about its potential role. Should PIPE-791 demonstrate significant efficacy in reducing disease progression with an acceptable safety profile, it could fulfill a critical niche in the treatment of Progressive MS. While current therapies like Ocrelizumab and Siponimod have made strides in managing this condition, the introduction of PIPE-791 could provide a much-needed alternative approach, particularly for patients who have limited response to existing treatments or who experience significant side effects.
Preclinical data
The preclinical data for PIPE-791, a novel LPA1R antagonist, presents a promising avenue for the treatment of Progressive Multiple Sclerosis (MS) by targeting the chronic demyelination and neuroinflammation associated with the disease. Here's a comprehensive summary of the key studies and results:
-
PIPE-791 Overview: As a high affinity, orally available, brain-penetrant small molecule, PIPE-791 demonstrates significant potential in modifying disease progression through promoting oligodendrocyte precursor cell (OPC) differentiation into oligodendrocytes and enhancing oligodendrocyte survival in inflammatory environments. Its effectiveness in reversing immune-mediated neuroinflammation and promoting remyelination was observed in both in vivo and in vitro MS models.
-
LPA1R Expression in OPCs: Independent assessments confirmed enriched LPA1R expression in OPCs compared to other isoforms (LPA2-5), providing a targeted approach for PIPE-791's mechanism of action.
-
Rodent OPC Differentiation: In vitro studies with rodent OPCs showed a concentration-dependent increase in differentiation into oligodendrocytes upon treatment with PIPE-791, with an EC50 (effective concentration for 50% of its maximum response) of 108 nM.
-
Remyelination in Organotypic Brain Slice Culture: Ex vivo studies demonstrated that PIPE-791 induces remyelination in rodent cortical brain slices after demyelinating insults, with an EC50 of 74 nM.
-
Oligodendrocyte Survival: In vitro experiments revealed that PIPE-791 provides dose-dependent protection of oligodendrocytes from death induced by inflammatory cytokines TNFα and IFNγ, with an EC50 of 119 nM, highlighting its potential in mitigating neuroinflammation-associated cell death.
-
Effectiveness in Human OPCs: Extending beyond rodent models, PIPE-791 was shown to induce differentiation of OPCs into mature oligodendrocytes in human cortical slice cultures, suggesting its applicability in human settings.
-
Inhibition of LPA-Induced Microglia Activation: PIPE-791 was effective in inhibiting LPA-induced microglial activation in vitro, a crucial step in reducing the inflammatory environment that impedes remyelination.
In Vivo CNS LPA1R Occupancy
-
[3H]-PIPE-497 Radioligand Binding: A novel selective LPA1 radioligand was used to assess the receptor occupancy of PIPE-791 in rodents. Following four days of once-daily oral administration, PIPE-791 showed a dose-dependent inhibition of radioligand binding.
-
Effective Dosing: The ED50 (the dose effective in 50% of the population) was found to be 0.03 mg/kg. Plasma EC50 (concentration for 50% maximal effect) and EC90 (concentration for 90% maximal effect) were 9 ng/mL (19 nM) and 31 ng/mL (65 nM), respectively. After correcting for plasma protein binding, the unbound EC50 is estimated to be 0.7 nM.
-
Therapeutic Implications: These results are utilized to inform pharmacokinetics (PK) and potential human dosing strategies, demonstrating effective CNS receptor occupancy at relatively low doses.
EAE Rodent Model: Remyelination, Neuroinflammation, and Neuronal Function
-
Model of MS Pathology: The experimental autoimmune encephalomyelitis (EAE) model in rodents, which mimics key features of MS including inflammation, demyelination, and axonal loss, was used.
-
Efficacy of PIPE-791: Administration of 3 mg/kg PIPE-791 resulted in significant improvements:
- Increased Myelinated Axons: A statistically significant increase in the percentage of myelinated axons in the optic nerve was observed compared to vehicle-treated controls.
- Restoration of VEP Latency: Visual evoked potentials (VEP) latency was restored, indicating improved neuronal function.
- Reduction in Neuroinflammation: A decrease in IBA1+ cells was noted, signifying reduced neuroinflammation.
- Statistical Significance: The improvements in VEP latency, axonal myelination, and neuroinflammation were all statistically significant.
LPS-Induced Neuroinflammation Model
-
Rodent LPS Challenge: PIPE-791's effect on neuroinflammation was further evaluated in a rodent model where neuroinflammation was induced by lipopolysaccharide (LPS).
-
Cytokine Reduction: A single oral dose of PIPE-791 significantly reduced the expression of several neuroinflammatory cytokines and chemokines, including Cxcl1, Cxcl10, Ccl5, and Il1b, following LPS challenge.
-
Broader Anti-inflammatory Effect: While reductions in Il6 and Tnfa expressions were observed, statistical significance was not reached, suggesting a broad but nuanced anti-inflammatory effect of PIPE-791.
The preclinical findings for PIPE-791 are compelling and suggest that this novel LPA1R antagonist could offer significant therapeutic benefits in the treatment of idiopathic pulmonary fibrosis and progressive multiple sclerosis (MS). Let's delve into the significance of these findings, the predictive validity and translatability of the disease models used, and consider the differences between these models and human biology.
-
Significance of Findings
- Promotion of OPC Differentiation and Remyelination: The ability of PIPE-791 to promote differentiation of oligodendrocyte precursor cells (OPCs) into mature oligodendrocytes and enhance remyelination is particularly noteworthy. In diseases like MS, loss of oligodendrocytes and the consequent demyelination are central to disease pathology. Therapeutic strategies that can effectively promote remyelination are of high interest, as they can potentially reverse this pathology and improve neurological function.
- Neuroinflammation Reduction: The demonstrated capability of PIPE-791 to reduce neuroinflammation, both in vitro and in vivo, addresses another critical aspect of MS pathology. Neuroinflammation is a hallmark of MS and contributes to the progression of neurodegeneration. By inhibiting this process, PIPE-791 may slow or halt disease progression.
- CNS Receptor Occupancy: The findings related to CNS LPA1R occupancy are crucial for understanding the drug’s mechanism of action and for guiding dosing strategies in future clinical trials. High receptor occupancy in the CNS, achieved at relatively low doses, suggests that PIPE-791 has excellent brain penetration and effective target engagement.
-
Predictive Validity and Translatability
- Disease Models Used: The experimental autoimmune encephalomyelitis (EAE) model and the lipopolysaccharide (LPS)-induced neuroinflammation model are widely used in MS research. The EAE model, in particular, is considered to closely mimic human MS in terms of immunopathological and neuropathological mechanisms, offering high predictive validity for therapeutic interventions aimed at reducing inflammation and promoting remyelination.
- Translatability to Human Disease: While these models provide valuable insights, it's important to recognize limitations in their translatability to human disease. The EAE model, for instance, is an induced model of disease and, while it shares many pathological features with MS, does not fully replicate the human condition. Differences in immune system function, CNS biology, and disease progression between rodents and humans can affect translatability. However, the use of human organotypic brain slice cultures in assessing PIPE-791's effects on OPC differentiation enhances translational relevance.
-
Model Systems vs. Human Biology
- Species-Specific Differences: There are inherent differences in the biology of model organisms compared to humans, including differences in the immune system, CNS environment, and molecular targets. Such differences can impact the efficacy and safety profile of a drug when transitioning from preclinical models to human trials.
- Predictive Limitations: While preclinical models are essential for understanding disease mechanisms and evaluating potential therapies, they cannot fully predict the complex interplay of factors influencing drug efficacy and safety in humans. This is particularly true for diseases with multifactorial etiologies like MS.
In conclusion, the preclinical data for PIPE-791 are promising and justify further development, including clinical trials in humans. The promotion of OPC differentiation, remyelination, and reduction of neuroinflammation are highly relevant to treating MS. However, the transition from preclinical models to clinical efficacy in humans will require careful consideration of the differences between these models and human biology, and the potential need for innovative trial designs to adequately test PIPE-791's therapeutic potential.
Clinical trial overview
Study Design Summary of PIPE-791
Overview:
The study investigating PIPE-791, aimed at treating progressive multiple sclerosis, is structured in three parts, involving a Single Ascending Dose (SAD), Multiple Ascending Dose (MAD), and a food effect evaluation on a selected SAD cohort. This phase 1 trial, designed as a randomized, double-blind intervention, involves both PIPE-791 and placebo treatments to assess safety, tolerability, and pharmacokinetics in healthy volunteers.
Details of Design:
- Part 1 (SAD): Approx. 48 subjects, 6-week duration.
- Part 2 (MAD): Approx. 32 subjects, 7-week duration.
- Part 3 (Food Effect): Approx. 8 subjects, 6-week duration.
The intervention involves escalating doses of PIPE-791, with assessments for safety through various clinical and laboratory measures, including TEAEs, electrocardiograms, and vital statistics.
Critiques of the Study Design:
- Population: The trial involves healthy volunteers, which is standard for Phase 1 studies focused on safety and pharmacokinetics. However, since the target condition is progressive multiple sclerosis, transitioning to clinical efficacy in patients with this condition will be crucial in subsequent phases.
- Sample Size: Given the complexity and variability of drug responses, the relatively small sample sizes, especially for Part 3 involving the food effect, might limit the generalizability of the findings.
- Duration: The short duration of each part may not fully capture long-term safety concerns or the full pharmacokinetic profile of PIPE-791, particularly for conditions like progressive multiple sclerosis that require long-term treatment strategies.
- Blinding and Randomization: While the study benefits from triple blinding and randomization, ensuring bias minimization, the outcomes heavily depend on the accuracy and consistency of the blinding process.
Operational and Technical Challenges:
- Recruitment and Retention: Recruiting a sufficient number of healthy volunteers willing to partake in a study with escalating doses of an experimental drug presents challenges. Moreover, retaining participants throughout the entire study duration, especially for the MAD phase, may prove difficult.
- Dosing Strategy: Determining the appropriate escalating doses, which are safe yet high enough to provide meaningful data, requires careful planning and monitoring to avoid adverse effects while ensuring the study's scientific value.
- Data Quality: Ensuring consistent, high-quality data collection across all study parts, especially with complex assessments like cardiac repolarization measures and pharmacokinetic analyses, requires meticulous execution and rigorous quality control.
- Food Effect: Evaluating the food effect on drug bioavailability entails carefully controlled conditions and dietary standardization, which can be challenging to enforce and may introduce variability in the data.
In summary, while the study design of PIPE-791 offers a structured approach to evaluating this promising drug for progressive multiple sclerosis, it faces typical Phase 1 challenges, including population representation, sample size limitations, and operational complexities that need careful management to ensure the study's success and relevance to the target patient population.
Appropriateness of Primary and Secondary Endpoints
- Primary Endpoint (Safety): For a Phase 1 trial, focusing on safety through the measurement of treatment-emergent adverse events (TEAEs) is appropriate. This will ascertain if PIPE-791 is safe for further investigation in targeted patient populations.
- Secondary Endpoints (Cardiac Safety and Pharmacokinetics): Monitoring changes in cardiac repolarization and assessing the pharmacokinetics of PIPE-791 through blood and urine concentrations are also suitable for evaluating the drug's safety profile and how it's metabolized and excreted. These are standard and crucial metrics that can influence dosing regimens in subsequent trials.
Inclusion/Exclusion Criteria
- Age and Health Status: The criteria limit participants to a relatively young and healthy demographic (18-55 years old), which reduces the variability caused by comorbidities and polypharmacy but also limits the generalizability of the results to the older MS population, who often demonstrate a higher prevalence of progressive MS.
- Reproductive Precautions: The requirement for double-barrier contraceptive methods is standard for controlling risks associated with potential drug-induced teratogenic effects.
- Exclusion of Confounding Factors: Excluding individuals with recent significant illnesses, chronic diseases, a history of substance abuse, significant blood donation, or recent participation in other studies helps minimize confounding variables that could obscure the drug's safety profile and pharmacokinetics.
Reproducibility Challenges
Population Representativeness: The strict age criteria and requirement for medical health may challenge the reproducibility of these results in the broader, more diverse MS population, including older patients and those with comorbid conditions typical of progressive MS demographics.
Lifestyle and Medication Restrictions: Excluding individuals based on recent medication use or specific lifestyle factors (e.g., alcohol intake) may also limit enrollment and the applicability of findings to real-world settings where patients may not adhere to such stringent criteria.
Interpreting Findings in MS Context: Given this study's healthy volunteer base, translating these data to assess efficacy or predict safety in the MS population will require carefully designed phase 2 and phase 3 trials that consider the disease's pathology.
In summary, while this study is appropriately designed to evaluate the safety and pharmacokinetics of PIPE-791 in a controlled setting, its criteria and design pose challenges for directly extrapolating findings to the progressive MS population. The study sets a necessary foundation, but subsequent research must specifically address the unique characteristics and needs of MS patients to fully validate PIPE-791's potential efficacy and safety in this group.
Clinical trial results
The clinical data from PIPE-791's Phase 1 trial in healthy volunteers can be summarized as follows:
- Study Design: The trial was a single-center, double-blind, placebo-controlled trial. It evaluated the safety, tolerability, and pharmacokinetics (PK) of PIPE-791 administered orally to healthy male and female volunteers aged 18 to 55 years.
- Dosing Groups:
- The Single Ascending Dose (SAD) part included four dose cohorts (1 mg, 5 mg, 10 mg, and 20 mg) with six participants in each cohort, and an additional eight participants receiving a placebo.
- The Multiple Ascending Dose (MAD) part included three dose cohorts (1 mg, 3 mg, and 10 mg) with six participants in each cohort and an additional six receiving a placebo.
- The 1 and 3 mg MAD cohorts received daily doses for 7 days, while the 10 mg MAD cohort received daily doses for 14 days.
- The food effect was tested by administering the 10 mg dose in both fasted and fed states to the SAD cohort.
- Safety and Tolerability: PIPE-791 was well-tolerated across all dose cohorts. Most treatment-emergent adverse events (TEAEs) were Grade 1 in severity, with two Grade 2 AEs reported under the active drug (back pain in SAD4, constipation in MAD3) and one Grade 2 AE (headache) in a placebo subject. No Grade 3 or 4 AEs were reported.
- Adverse Events (AEs): Excluding AEs related to venipuncture soreness and EKG contact dermatitis, there was no apparent relationship or pattern between AEs and the administered doses. All AEs recovered without sequelae.
- Pharmacokinetics:
- Half-life: It was dose-dependent, ranging from 55 hours for the 1 mg dose to 31 hours for the 20 mg dose, indicating a faster elimination rate at higher doses.
- Food Effect: Co-administration with food delayed the time to reach maximum concentration (Tmax) and reduced the maximum concentration (Cmax), but did not affect the overall exposure to the drug.
- Dose-Responsiveness: There was a clear dose-dependent relationship in drug concentration, with higher doses resulting in higher blood concentrations.
- Drug Accumulation: The concentrations on the seventh day for the 1 mg and 3 mg MAD cohorts were slightly higher than those on the first day, suggesting some drug accumulation with multiple dosing.
- Efficacy: The trial met both primary (safety and tolerability) and secondary (PK profile) objectives.
In conclusion, the Phase 1 trial data suggest that PIPE-791 has a favorable safety profile and a predictable pharmacokinetic behavior that is dose-responsive and influenced by food intake. The pharmacokinetics showed a faster clearance at higher doses, with some accumulation on repeated dosing for the lower doses. The study met its stated primary and secondary endpoints.
PIPE-307
Scientific background
The therapeutic rationale for using an M1 muscarinic receptor (M1R) antagonist in the treatment of relapse-remitting multiple sclerosis (RRMS) and depression is anchored in the complex interactions between the cholinergic system (of which M1R is a part) and both the immune system and neural circuitry, which are dysregulated in these conditions.
Relapse-Remitting Multiple Sclerosis (RRMS)
- Immunomodulation: The cholinergic system, through the "cholinergic anti-inflammatory pathway", plays a crucial role in regulating the immune response. M1Rs are expressed in various immune cells, and their modulation can influence the inflammatory processes central to RRMS pathology. By antagonizing M1R, the aim is to downregulate the pro-inflammatory responses, potentially reducing the frequency and severity of relapses in RRMS by preventing immune cells from attacking myelin in the central nervous system.
- Neuroprotection: M1R antagonists might contribute to neuroprotection by modulating glutamate release and reducing excitotoxicity, a condition that leads to neuron damage and is implicated in the pathogenesis of multiple sclerosis. This neuroprotective effect could help in slowing down the progression of RRMS.
Depression
- Neurotransmitter Regulation: M1Rs play a significant role in modulating neurotransmitter systems, including those involved in mood regulation such as serotonin and dopamine. M1R antagonists could help in restoring the balance in neurotransmitter levels, thereby alleviating symptoms of depression.
- Neuroplasticity: Chronic depression has been associated with reduced neuroplasticity, leading to cognitive deficits and emotional disturbances. M1R antagonists may promote neuroplasticity by enhancing synaptic plasticity and promoting the growth of neural connections. This can result in improved mood, cognition, and overall brain health in individuals with depression.
- Stress Response Modulation: M1R is involved in the hypothalamic-pituitary-adrenal (HPA) axis regulation, which controls the stress response. Dysregulation of the HPA axis is a known factor in the development of depression. Through M1R antagonism, there might be a potential to restore HPA axis function, thus providing a therapeutic benefit in treating depression.
In summary, the use of an M1R antagonist provides a multi-faceted approach in the treatment of RRMS and depression. For RRMS, the rationale centers around immunmodulation and potential neuroprotection. For depression, the focus is on neurotransmitter regulation, enhancing neuroplasticity, and modulating the stress response. This dual action on both the immune system and brain function makes M1R antagonists promising therapeutic candidates for these conditions. Further clinical research is necessary to fully understand their efficacy and safety profile in these specific patient populations.
The science behind the use of M1 muscarinic receptor (M1R) antagonists in the treatment of relapse-remitting multiple sclerosis (RRMS) and depression is still emerging, and while promising, it encompasses areas of both established research and ongoing debate. Here's an overview of the current state of the science, the uncertainties, and the level of evidence for the processes described:
Immunomodulation in RRMS
- Established Science: The cholinergic anti-inflammatory pathway is a well-documented phenomenon showing the cholinergic system's role in regulating immune responses. Several studies have confirmed the existence of this pathway and its potential implications for autoimmune diseases like multiple sclerosis (MS).
- Uncertainties/Debate: The direct application of M1R antagonism as a therapeutic strategy for RRMS is less established. The precise mechanisms through which M1R antagonists could modulate immune responses specifically in the context of RRMS remain to be fully elucidated. There is ongoing research into whether targeting M1R alone is sufficient or if a broader approach targeting multiple receptors might be more effective.
Neuroprotection
- Established Science: The concept of excitotoxicity contributing to neuronal damage in MS is well-supported by current research. There is also evidence for the involvement of the muscarinic receptors in modulating neurotransmitter release, including glutamate.
- Uncertainties/Debate: The neuroprotective potential of M1R antagonists specifically within the pathology of MS is a subject of current research. The balance between inhibiting harmful excitotoxicity without disrupting normal neurotransmitter functions is complex and not yet fully understood.
Neurotransmitter Regulation in Depression
- Established Science: Imbalances in neurotransmitter systems, such as serotonin and dopamine, are well-established factors in the pathology of depression. The role of the cholinergic system in mood regulation is also supported by extensive research.
- Uncertainties/Debate: While targeting the cholinergic system, and M1R specifically, as a strategy for depression shows promise, clinical evidence directly linking M1R antagonism to improved outcomes in depression is still developing. The complexity of depression means that no single neurotransmitter pathway is likely to be a panacea, and how M1R antagonists fit into the broader treatment landscape remains an open question.
Neuroplasticity and Stress Response Modulation
- Established Science: The effects of depression on reducing neuroplasticity and the dysregulation of the HPA axis are well-documented.
- Uncertainties/Debate: The ability of M1R antagonists to positively influence neuroplasticity and modulate the HPA axis specifically for therapeutic benefit in depression is promising but still in the early stages of research. This area is particularly challenging because of the subjective nature of depression symptoms and the difficulty in quantifying improvements in neuroplasticity and HPA axis function.
Overall Level of Evidence
The therapeutic rationale for M1R antagonists in RRMS and depression is grounded in a combination of established science and areas of active investigation. The overall level of evidence varies, with a solid foundation in understanding the basic functions of M1R but less clarity on the direct clinical implications of M1R antagonism in these specific conditions. Current research is promising but must progress from preclinical studies to more extensive clinical trials to validate these potential therapies' efficacy and safety. The theory and preliminary data suggest potential, but more definitive evidence is needed to turn this theoretical understanding into practical treatments.
Direct clinical trial results and literature specifically linking M1 muscarinic receptor (M1R) antagonism to therapeutic effects in relapse-remitting multiple sclerosis (RRMS) and depression are limited and still emerging. However, I can provide a synthesis of related findings that support the theoretical basis for considering M1R antagonists in these conditions. It's important to note that the scientific community constantly generates new data, so recent publications might offer more direct evidence.
Literature Supporting M1R's Role in RRMS
- Immunomodulatory Role: While specific studies directly associating M1R antagonism with RRMS treatment are sparse, research on the cholinergic anti-inflammatory pathway provides a foundation for this therapeutic approach. For instance, studies on the vagus nerve's role in modulating immune responses suggest that acetylcholine, acting through muscarinic receptors, can influence immune cell activity and inflammation (Tracey, K.J., 2009, "Reflex control of immunity," Nature Review Immunology). Given M1R's prominent role in the cholinergic system, these findings underpin the hypothesis that M1R antagonists could modulate immune responses in RRMS.
- Neuroprotection and Excitotoxicity: Research into the mechanisms of neurodegeneration in MS highlights the role of excitotoxicity, which is partly mediated by glutamate receptors. The potential for M1R antagonists to modulate glutamate release suggests a therapeutic avenue worth exploring for neuroprotection in MS (Newcombe, J., et al., 2018, "Glutamate and MS," Journal of the Neurological Sciences).
Literature Supporting M1R's Role in Depression
- Neurotransmitter Balance: The cholinergic system’s involvement in mood disorders is supported by evidence linking acetylcholine's dysregulation to symptoms of depression (Janowsky, D.S., et al., 1972, "Cholinergic reversal of manic symptoms," Lancet). While these studies focus more broadly on the cholinergic system rather than M1R specifically, they provide a backdrop for investigating specific cholinergic receptors like M1R.
- Neuroplasticity: The theory that M1R antagonists could enhance neuroplasticity and contribute to antidepressant effects finds some support in research on neurotrophic factors, like brain-derived neurotrophic factor (BDNF), which are involved in neuroplasticity and are affected by cholinergic signaling (Caughlan, A., et al., 2004, "Cholinergic modulation of neuroplasticity," Brain Research Reviews).
- Stress Response: Studies on the HPA axis and its dysregulation in depression sometimes reference the broader cholinergic system's role in stress responses (Nemeroff, C.B., and Vale, W.W., 2005, "The neurobiology of depression: inroads to treatment and new drug discovery," Journal of Clinical Psychiatry). The extrapolation to M1R's specific involvement is part of ongoing research, emphasizing a need for more direct studies.
Conclusion
Although direct clinical evidence specifically targeting the M1R in RRMS and depression is still developing, there is a substantial indirect rationale based on broader research into the cholinergic system’s role in these diseases. The areas of immunomodulation, neuroprotection, neurotransmitter balance, neuroplasticity, and stress response modulation all point toward potential therapeutic roles for M1R antagonists. Given the complexity of RRMS and depression, more focused research and clinical trials are necessary to translate these theoretical foundations into practical, evidence-based treatments.
When evaluating the therapeutic rationale for using M1 muscarinic receptor (M1R) antagonists in treating relapse-remitting multiple sclerosis (RRMS) and depression, it's critical to acknowledge the strengths and weaknesses within the existing evidence base.
Strengths of the Evidence Base
- Broad Understanding of the Cholinergic System: There's a well-established body of research that outlines the roles of the cholinergic system in immune modulation, neurotransmission, and neuroplasticity. This foundational knowledge supports targeting the cholinergic system, including M1R, for therapeutic purposes.
- Animal Models and Preclinical Studies: Research using animal models and preclinical studies often demonstrates the potential benefits of modulating the cholinergic system in contexts relevant to RRMS and depression, such as inflammation, neuroprotection, and neurotransmitter balance. These studies form a basis for the hypothesized benefits of M1R antagonism.
- Links Between Neurotransmitter Dysregulation and Disease: For depression, in particular, the link between neurotransmitter dysregulation (including the cholinergic system) and mood disorders is well-established. This connection provides indirect support for exploring M1R antagonists as a therapeutic avenue.
Weaknesses of the Evidence Base
- Lack of Direct Clinical Data: One of the most significant weaknesses is the limited direct clinical evidence for M1R antagonists' efficacy in RRMS and depression. Much of the current understanding is extrapolated from broader studies on the cholinergic system rather than specific trials involving M1R antagonists.
- Complexity of Conditions: Both RRMS and depression are complex, multifactorial diseases. The therapeutic rationale often simplifies these conditions to fit the mechanism of action being studied. This reductionist approach may overlook the complexity of the diseases and the multifaceted influence of the cholinergic system.
- Potential Side Effects and Safety Profile: The cholinergic system influences various physiological functions, raising concerns about the potential side effects of M1R antagonists. The broad effects of muscarinic antagonism may translate into adverse outcomes, and the existing evidence base may not fully account for these risks.
- Variability in Response: The heterogeneity among individuals with RRMS or depression implies that responses to M1R antagonists might vary significantly. The current evidence base might not adequately capture this variability or predict who might benefit most from such treatments.
Summary
The proposal to use M1R antagonists for RRMS and depression is underpinned by solid theoretical and preclinical rationales related to the cholinergic system's roles in immunomodulation, neurotransmitter regulation, and neuroprotection. However, this theoretical support meets practical challenges in the form of limited direct clinical evidence, the inherent complexity of the target conditions, concerns about side effects, and patient variability. Future research, particularly well-designed clinical trials, is essential to strengthen the evidence base, clarify the therapeutic potential of M1R antagonists, and pinpoint their role in treatment protocols for RRMS and depression.
Market overview
Relapse remitting multiple sclerosis
Relapse-Remitting Multiple Sclerosis (RRMS) is the most common form of Multiple Sclerosis (MS), a chronic autoimmune disease affecting the central nervous system (CNS), which comprises the brain and spinal cord. Here's an overview based on scientific and medical literature:
Pathology
In RRMS, the immune system mistakenly attacks myelin, the protective sheath covering nerve fibers, causing inflammation and damage. This damage leads to scar tissue or lesions, interfering with the transmission of nerve signals. The pathology is marked by phases of acute attacks (relapses) followed by periods of partial or complete recovery (remissions).
Symptoms
Symptoms of RRMS vary widely among patients, depending on the location and extent of the nerve damage. Common symptoms include:
- Fatigue
- Difficulty walking
- Numbness or weakness in one or more limbs
- Electric shock sensations with certain neck movements (Lhermitte's sign)
- Tremors, lack of coordination, or unsteady gait
- Vision problems, including blurred or double vision
- Slurred speech
- Bladder and bowel dysfunction
Prognosis
The prognosis of RRMS can be highly variable. While it is a lifelong condition with no cure, treatments can help manage symptoms, reduce the number of relapses, and slow the progression of the disease. The majority of individuals with RRMS eventually transition to a secondary progressive course (SPMS) in which the disease worsens more steadily, with or without relapses.
Management and Treatment
Management strategies for RRMS focus on recovery from relapses, slowing disease progression, and managing symptoms. Treatment options include:
- Disease-modifying therapies (DMTs): These medications can reduce the frequency and severity of relapses and slow disease progression.
- Corticosteroids: Often used to reduce inflammation and shorten the duration of relapses.
- Symptomatic treatments: Medications and therapies to manage symptoms such as muscle spasticity, fatigue, and bladder or bowel control problems.
- Rehabilitation: Physical therapy, occupational therapy, and speech therapy can help manage symptoms and improve quality of life.
Living with RRMS
Living with RRMS requires adapting to changes in health, managing symptoms, and receiving continuous treatment to maintain as normal a life as possible. Regular follow-up with healthcare providers, a healthy lifestyle, and support from friends, family, and MS support groups can play crucial roles in managing the disease.
Given the context of Relapse-Remitting Multiple Sclerosis (RRMS) and the information provided about PIPE-307, it's clear there's a considerable market opportunity for novel therapies in this domain. Let's explore the potential market for PIPE-307 in the context of the current treatment landscape, successful drugs, the standard of care, and unmet medical needs in RRMS.
Current Treatment Landscape
Current treatments for RRMS include a range of Disease-Modifying Therapies (DMTs) such as interferon-beta products (e.g., Avonex, Rebif), glatiramer acetate (Copaxone), and newer oral agents like fingolimod (Gilenya), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio). Several monoclonal antibodies such as natalizumab (Tysabri) and ocrelizumab (Ocrevus) have also been approved, offering high efficacy in reducing relapses and slowing disease progression.
Standard of Care and Successful Drugs
The standard of care in RRMS involves early initiation of DMTs to control the disease activity and manage symptoms. Drugs like Ocrevus have redefined treatment standards due to their efficacy in both RRMS and primary progressive MS. However, treatment decisions are highly individualized, considering factors like disease activity, patient preferences, and potential side effects.
Unmet Medical Needs
Despite the availability of multiple DMTs, significant unmet needs remain in RRMS:
- Efficacy: Not all patients respond well to existing therapies, and some experience breakthrough disease activity.
- Safety and Tolerability: Side effects and long-term safety concerns of current DMTs lead to discontinuation or switching therapies.
- Convenience: The need for frequent dosing or infusion treatments impacts patient compliance and quality of life.
- Progression Prevention: Most available treatments focus on relapse rate reduction but are less effective in preventing the progression of disability.
Market Opportunity for PIPE-307
PIPE-307, a novel, small molecule selective inhibitor of the muscarinic type 1 (M1R), presents a unique positioning in the RRMS market due to its mechanism of action and the convenience of being an oral therapy. Its development addresses several unmet needs by potentially offering a new, well-tolerated option that could improve patient adherence and quality of life, straightforward dosing, and potentially a new mechanism to manage the disease.
Given that PIPE-307 is described as the most clinically advanced selective M1R antagonist and is in development in collaboration with a major pharmaceutical company (J&J), this suggests strong backing and confidence in its potential. The focus will likely be not just on efficacy in preventing relapses but also on safety, tolerability, and the impact on disease progression and neurodegeneration.
To capitalize on the market opportunity, PIPE-307 will need to demonstrate clear benefits over existing therapies in its clinical trials, focusing on efficacy, safety, and patient-reported outcomes like convenience and quality of life. Its differentiation could come from its novel mode of action targeting the muscarinic receptors, which have not been extensively targeted in RRMS, potentially providing benefits not seen with other treatments.
The competitive landscape for RRMS is becoming increasingly crowded with novel mechanisms of action being explored. PIPE-307's success will depend on its ability to offer distinct advantages over these emerging therapies, whether through superior efficacy, better safety and tolerability, easier administration, or a combination of these factors. The development strategy for PIPE-307 should closely monitor evolving treatment paradigms and patient and physician expectations to ensure it meets the current and future needs of the RRMS community. As the landscape evolves, staying abreast of these developments and adapting to the changing market will be key to achieving and maintaining a competitive edge.
The treatment landscape for Relapse-Remitting Multiple Sclerosis (RRMS) has expanded significantly over the past decade, introducing several notable drugs. These treatments aim to reduce the frequency and severity of relapses, slow the progression of the disease, and manage symptoms, ultimately improving the quality of life for patients. Here is an overview of some key medications, including newer, recently approved options:
- Natalizumab (Tysabri)
Natalizumab is a highly effective monoclonal antibody administered via infusion. It works by blocking the migration of potentially damaging immune cells from the bloodstream, across the blood-brain barrier, into the brain and spinal cord. Tysabri is particularly favored for patients with highly active RRMS due to its efficacy but carries the risk of a rare, serious brain infection known as progressive multifocal leukoencephalopathy (PML). - Fingolimod (Gilenya)
Gilenya was the first oral disease-modifying therapy (DMT) approved for RRMS. It works by sequestering lymphocytes (a type of white blood cell involved in the immune response) in lymph nodes, preventing them from reaching the central nervous system and causing damage. Gilenya is associated with a risk of heart rate issues, making the first dose monitoring essential. - Ocrelizumab (Ocrevus)
Approved for both RRMS and Primary Progressive MS (a less common form of the disease), Ocrevus targets CD20-positive B cells, which are believed to play a key role in the myelin and neuron damage characteristic of MS. It is administered via infusion and has been associated with a significant reduction in relapse rates and slowing disease progression in RRMS. - Dimethyl Fumarate (Tecfidera)
Tecfidera is an oral medication that reduces inflammation and protects against damage to the brain and spinal cord. While its exact mechanism is not fully understood, it's thought to have antioxidant properties that protect against cell damage. Common side effects include flushing and gastrointestinal issues. - Teriflunomide (Aubagio)
Another oral DMT, Aubagio, is believed to work by inhibiting the proliferation of active immune cells that attack the myelin sheath in the central nervous system. It is generally well-tolerated but can cause liver damage and is not recommended for pregnant women due to the risk of birth defects. - Ofatumumab (Kesimpta)
Approved in August 2020, Kesimpta is a once-monthly self-injected treatment for RRMS. It targets CD20-positive B cells like Ocrevus but offers the convenience of at-home injections rather than infusions. It has shown efficacy in reducing relapses and is generally well-tolerated. - Ponesimod (Ponvory)
Approved in March 2021, Ponvory is an oral S1P receptor modulator, similar in mechanism to Gilenya but with potentially fewer cardiac side effects. It works by trapping immune cells in lymph nodes, reducing their ability to reach and damage the CNS. Ponvory is indicated for the treatment of adults with RRMS and has shown a favorable safety profile.
These drugs represent a selection of the key treatments available for RRMS, highlighting the shift towards more targeted therapies that offer patients both efficacy and convenience. The choice of treatment is highly individualized, taking into account disease activity, lifestyle considerations, and potential side effects. As research advances, new therapies continue to emerge, providing hope for even more effective management of RRMS in the future.
Although specific details about PIPE-307's mechanism of action, efficacy, safety, and tolerability profiles have not been provided in this context, we can infer its potential place in the standard of care for Relapse-Remitting Multiple Sclerosis (RRMS) based on the current treatment landscape and the information on PIPE-307 being a novel, small molecule selective inhibitor of the muscarinic type 1 (M1R) receptor in development for RRMS.
The treatment landscape of RRMS is characterized by a diverse array of Disease-Modifying Therapies (DMTs), including injectables (e.g., interferon-beta products), oral medications (e.g., dimethyl fumarate, fingolimod), and infusion therapies (e.g., natalizumab, ocrelizumab). Each of these therapies is aimed at reducing relapse rates, slowing disease progression, and managing symptoms, yet they come with varying efficacy, safety profiles, and dosing regimens. Despite the availability of these treatments, there remains an unmet need for therapies that offer improved efficacy, fewer side effects, and greater convenience.
Potential Position of PIPE-307 in the Standard of Care
Novel Mechanism of Action: By selectively inhibiting the M1R receptor, PIPE-307 could modulate immunological and neurological pathways in a novel way that may complement or offer advantages over existing mechanisms, especially for patients who have an inadequate response to current therapies.
Safety and Tolerability: If PIPE-307 demonstrates a favorable safety and tolerability profile in ongoing and future clinical trials, it could become a preferred option for patients who experience intolerable side effects from existing DMTs.
Convenience and Adherence: As an oral medication, PIPE-307 could offer added convenience over infusion-based therapies, potentially improving adherence and patient satisfaction, especially if dosing frequency is competitive (e.g., daily or less frequent).
Efficacy: To secure a place in the RRMS treatment paradigm, PIPE-307 must demonstrate robust efficacy in reducing relapse rates and slowing disease progression. Superior efficacy compared to existing drugs, especially in patients with highly active RRMS or those who are treatment-resistant, could strongly position PIPE-307 in the market.
Regulatory Strategy and Market Access: Collaboration with a major pharmaceutical partner (e.g., J&J) could expedite development, regulatory approval, and market access. Moreover, strategically positioning PIPE-307 for specific patient segments based on its clinical profile could enhance its adoption.
PIPE-307 has the potential to address unmet needs in the RRMS treatment landscape by offering a novel mechanism of action, which, depending on its efficacy and safety profile, may provide an attractive option for patients and clinicians. Its success and fit into the standard of care will ultimately depend on clinical trial outcomes, especially comparative data with existing DMTs, and how well it addresses the balance of efficacy, safety, and patient convenience. As the treatment landscape for RRMS continues to evolve, innovations like PIPE-307 represent the ongoing efforts to improve patient outcomes and quality of life.
Preclinical data
RRMS
The therapeutic rationale for using M1R (muscarinic receptor 1) antagonism in treating relapsing-remitting multiple sclerosis (RRMS) is rooted in the critical role of remyelination in managing and potentially reversing the progressive disability associated with the disease. Remyelination is considered a highly promising approach for preventing the accumulation of permanent disability in demyelinating diseases like MS by addressing axonal dysfunction secondary to chronic demyelination.
The interest in M1R antagonism for remyelination in RRMS stems from observations made during extensive drug screening investigations, which identified antimuscarinic compounds, including clemastine (an FDA-approved H1 antihistamine with antimuscarinic properties), as potential remyelination agents. Clemastine's efficacy in enhancing myelination was initially demonstrated in rodent models of EAE (experimental autoimmune encephalomyelitis), a standard preclinical model for MS, through its high affinity for muscarinic receptors. Subsequent identification of M1R as the specific target of clemastine in oligodendrocyte precursor cells (OPCs) highlighted the molecular pathway through which antimuscarinic compounds could facilitate remyelination.
The potential of M1R antagonism in promoting remyelination was further substantiated by the results of the ReBuild trial, a double-blind, randomized, placebo-controlled crossover Phase 2 trial in patients with RRMS. This trial demonstrated the first evidence of remyelination through improvements in visual evoked potential (VEP) latency, along with a trend towards improvement in low contrast visual acuity—a measure of visual function known to be impaired in MS patients compared to healthy controls. These outcomes suggest a direct therapeutic effect of remyelination.
However, clemastine's clinical utility in RRMS is limited by its side effects, primarily due to H1 receptor blockade, which restricts the potential for higher, more effective dosing due to a narrow therapeutic window. This limitation underscores the need for more selective M1R antagonists, like PIPE-307, which could offer the remyelination benefits without the adverse effects associated with H1 receptor antagonism. Thus, the success of the ReBuild trial and the mechanistic insights into M1R's role in OPC differentiation and myelination provide a strong scientific and clinical foundation for pursuing M1R antagonism as a therapeutic strategy in RRMS, aiming for improved safety and efficacy profiles.
The preclinical data on PIPE-307, a novel, small molecule, selective inhibitor of M1 muscarinic receptor (M1R), demonstrate its strong potential in the treatment of depression and relapsing-remitting multiple sclerosis (RRMS) through its ability to promote remyelination. Here is a comprehensive summary of the key studies and results:
-
In Vitro Studies on Rodent and Human Cells
- OPC Differentiation: PIPE-307 was tested for its ability to promote oligodendrocyte precursor cell (OPC) differentiation into myelin basic protein-positive (MBP+) oligodendrocytes, which are essential for myelin formation. The results indicated a concentration-dependent increase in MBP+ oligodendrocytes with an EC50 of 38.6 nM, showing efficacy comparable to T3 (triiodothyronine), a known promoter of OPC differentiation.
- Remyelination in Rodent Brain Slices: Ex vivo studies using rodent brain slices treated with lysolecithin (Lyso) to induce demyelination showed that subsequent treatment with PIPE-307 led to an increase in MBP protein expression, suggesting remyelination.
- Human Brain Tissue Assays: Evaluation of PIPE-307 on human brain tissue demonstrated an increase in mature oligodendrocytes, as indicated by a rise in adenomatous polyposis coli clone 1 positive (CC-1+) cells after treatment. This suggests PIPE-307's potential in promoting OPC maturation and remyelination in human tissues.
-
In Vivo Studies in MS Models
- EAE Model for Remyelination and Neuronal Function Restoration: In vivo studies using a rodent experimental autoimmune encephalomyelitis (EAE) model, which simulates inflammatory demyelination akin to MS, showed that treatment with PIPE-307 restored visual evoked potential (VEP) N1 latency and significantly increased the percentage of myelinated axons in the central nervous system (CNS). These results indicate PIPE-307's ability to promote remyelination and restore neuronal function in a disease model of MS.
Key Takeaways
- PIPE-307 demonstrates significant potential for inducing OPC maturation, enhancing myelin-membrane wrapping in vitro, and promoting remyelination in vivo, accompanied by functional improvements in a disease model relevant to MS.
- The drug shows efficacy comparable to known treatments in promoting OPC differentiation and outperforms in aspects of remyelination and functional recovery in the EAE model.
- These preclinical findings suggest PIPE-307's promising role in treating conditions like depression and RRMS by targeting and inhibiting M1R to facilitate remyelination and repair of neural damage.
This data provides some support for the therapeutic hypothesis of M1R antagonism in RRMS:
Support for the Therapeutic Hypothesis
- Mechanism of Action: The data strongly support the hypothesis that M1R antagonism can promote remyelination. The specific targeting of M1R by PIPE-307, demonstrated to induce oligodendrocyte precursor cell (OPC) maturation and myelination in vitro and in vivo, aligns well with the pathophysiological needs in RRMS, where demyelination and subsequent axonal damage are key drivers of disease progression.
- Preclinical Efficacy: The demonstrated efficacy of PIPE-307 in promoting remyelination and functional recovery in animal models of MS (e.g., the EAE model) provides a solid foundation for its therapeutic potential. The ability to replicate the effects of known remyelination agents (like clemastine) without their associated side effects due to more selective receptor targeting further strengthens the case for PIPE-307.
- Translation Potential: The translation of remyelination effects from animal models to humans is always challenging. However, the prior clinical validation of M1R antagonism with clemastine in the ReBuild trial, showing improvement in VEP latency and trends towards improved visual functions, offers indirect but supportive evidence that the mechanism exploited by PIPE-307 could have similar, if not better, therapeutic effects in humans due to its selectivity and potentially better safety profile.
De-risking Clinical Development
- Efficacy De-risking: The detailed preclinical studies provide a strong rationale for the efficacy of PIPE-307, significantly de-risking the early phases of clinical development. The evidence of functional recovery in animal models is particularly compelling, suggesting that PIPE-307 could offer tangible benefits to RRMS patients.
- Safety De-risking: The selective antagonism of M1R by PIPE-307 potentially de-risks the safety profile, addressing the limitations observed with clemastine. By focusing on M1R without affecting H1 receptors, PIPE-307 may avoid the sedative side effects that limit clemastine's dosing and thereby its efficacy in humans.
- Regulatory and Development Pathway: The prior evidence from the ReBuild trial, albeit with a different molecule, provides a regulatory precedent and helps in defining biomarkers (e.g., VEP latency improvement) that can be used in clinical trials to demonstrate efficacy. This aspect simplifies the development path and offers a clearer route to demonstrating clinical benefit.
Remaining Risks and Considerations
- Translation to Human Efficacy: Despite preclinical efficacy, the translation to humans remains a significant hurdle. Differences in the biology of remyelination between rodents and humans can lead to unexpected outcomes in clinical trials.
- Long-term Safety and Efficacy: Long-term safety and the durability of remyelination effects in humans are yet to be demonstrated. Potential compensatory mechanisms due to chronic M1R antagonism could emerge with prolonged treatment.
- Biomarker Validation: While improvements in VEP latency serve as a useful biomarker for remyelination, the clinical significance of these changes in terms of long-term disability progression remains to be fully validated.
In summary, the data supporting PIPE-307's development in RRMS significantly de-risk its clinical development plan, especially concerning mechanism-based efficacy and safety. However, successful translation to human efficacy, long-term safety, and the clinical significance of biomarkers remain as areas to be addressed through carefully designed clinical trials.
Market Overview
Depression
Depression, clinically known as Major Depressive Disorder (MDD), is a common and serious mood disorder that affects how a person feels, thinks, and handles daily activities. It is characterized by persistent feelings of sadness, emptiness, or hopelessness, and a lack of interest in activities once enjoyed. Depression can lead to a variety of emotional and physical problems, significantly impairing an individual's ability to function at work and home.
Pathology
The exact cause of depression is not fully understood and is believed to be multifactorial, involving a combination of biological, psychological, and social sources of distress. Research suggests that these factors lead to changes in brain function, including altered activity of certain neural circuits in the brain. The neurochemical regulation, particularly of neurotransmitters like serotonin, norepinephrine, and dopamine, is central to the pathology of depression. Genetics also play a role, with individuals having a family history of depression being more susceptible to the condition.
Symptoms
- Persistent sad, anxious, or "empty" mood
- Feelings of hopelessness or pessimism
- Irritability
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies and activities
- Decreased energy or fatigue
- Difficulty concentrating, remembering, or making decisions
- Insomnia or oversleeping
- Appetite and/or weight changes
- Thoughts of death or suicide, or suicide attempts
- Aches or pains, headaches, cramps, or digestive problems without a clear physical cause and/or that do not ease even with treatment
Prognosis
The prognosis for depression varies widely among individuals and depends on the severity of the symptoms, the presence of co-existing mental or physical disorders, the individual's social support and coping mechanisms, and the appropriateness and effectiveness of treatment. Many people with depression respond well to treatment, which may include psychotherapy, medications, or a combination of the two. Some individuals might require long-term treatment to maintain control over symptoms.
Management and Treatment
Effective treatments for depression are available and include:
- Medications: Antidepressants can help to modify brain chemistry. There are several types of antidepressants, and it might take some trial and error to find the most effective with the fewest side effects.
- Psychotherapy: Also known as talk therapy or counseling, this treatment involves discussing one's condition and related issues with a mental health professional. Cognitive-behavioral therapy (CBT) is among the most effective forms for treating depression.
- Lifestyle Adjustments: Changes in lifestyle, such as regular exercise, a healthy diet, and sufficient sleep, can help manage depression symptoms.
- Brain Stimulation Therapies: In severe cases or when medications and psychotherapy are not effective, brain stimulation treatments like electroconvulsive therapy (ECT) or transcranial magnetic stimulation (TMS) may be explored.
Depression is a complex condition that impacts many aspects of an individual's life but can often be effectively managed with the right combination of treatments. Understanding and recognizing the early signs of depression are crucial for timely and effective management. If you or someone you know is experiencing symptoms of depression, seeking professional help is a critical first step towards recovery.
PIPE-307, as a novel, small molecule selective inhibitor of the muscarinic type 1 (M1R) receptor, presents an interesting and potentially impactful approach for the treatment of depression. The opportunity for its integration into the current market for depression treatments may be considered by assessing the landscape of existing medications, the standard of care, along with the unmet medical needs within this therapeutic area.
Current Landscape and Standard of Care for Depression
The standard of care for depression typically involves a combination of psychotherapy and pharmacotherapy. Commonly prescribed medications for depression include:
- Selective Serotonin Reuptake Inhibitors (SSRIs) like fluoxetine (Prozac), sertraline (Zoloft), and citalopram (Celexa).
- Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs) such as venlafaxine (Effexor) and duloxetine (Cymbalta).
- Atypical Antidepressants and other classes targeting different neurotransmitter systems, including bupropion (Wellbutrin) and mirtazapine (Remeron).
- Other treatments include Tricyclic Antidepressants (TCAs) and Monoamine Oxidase Inhibitors (MAOIs), although these are less commonly prescribed today due to their side effect profiles.
While these medications are effective for many, a significant proportion of patients do not achieve full remission. The STAR*D study, one of the largest and longest independent trials on the treatment of major depression, highlighted that only about one-third of patients achieve remission with their first medication, and each successive treatment attempt has a diminished rate of success. This underscores the considerable unmet need for novel antidepressants with different mechanisms of action and better efficacy, tolerability, and safety profiles.
Unmet Medical Need
- Treatment-Resistant Depression: A substantial number of patients do not respond adequately to existing therapies, necessitating the development of novel treatments with different mechanisms.
- Safety and Tolerability: Many current antidepressants have side effects that can affect compliance and patient quality of life, such as sexual dysfunction, weight gain, and sleep disturbances.
- Rapid Onset of Action: Most antidepressants take several weeks to show clinical benefits, creating a gap in care for immediate relief needs.
- Cognitive Symptoms: Depression is often associated with cognitive impairments, yet few treatments directly address this aspect.
Market Opportunity for PIPE-307
Given the M1 receptor's role in cognitive function and mood regulation, PIPE-307 has the potential to meet several unmet needs within the depression treatment landscape:
- Novel Mechanism of Action: If PIPE-307 demonstrates efficacy in modulating mood and cognitive symptoms through its action on the M1R, it could offer a new option for patients unresponsive to traditional therapies.
- Safety Profile: Assuming PIPE-307 has fewer or less severe side effects compared to existing treatments, it could improve adherence and patient satisfaction.
- Cognitive Benefits: A therapy that offers cognitive benefits in addition to mood improvement would be highly valuable, especially for patients whose depressive episodes include significant cognitive deficits.
Collaboration with J&J suggests PIPE-307 has garnered interest from major players in the pharmaceutical industry, which could support its development and commercialization efforts. Its application in treating RRMS in addition to depression highlights its potential versatility and broad applicability across multiple indications, which could enhance its value proposition.
In summary, the development and potential approval of PIPE-307 could represent a significant advancement in the treatment of depression, addressing several critical unmet needs and offering patients a novel therapeutic option that could improve overall treatment outcomes.
The landscape of depression treatment is evolving rapidly with several promising therapies under development that might compete with PIPE-307. It's worth mentioning that PIPE-307, as a novel selective inhibitor of the muscarinic type 1 (M1R) receptor, represents an innovative approach in treating depression. The M1R pathway involvement in cognitive processes and potential mood regulation presents a distinct mechanism compared to traditional antidepressants. However, its market position will ultimately depend on its efficacy, safety profile, and how it addresses unmet medical needs compared to both existing and emerging therapies. Here are several areas of development that could provide competitive options:
1. Ketamine and Esketamine
Ketamine, traditionally used as an anesthetic, has shown rapid-acting antidepressant effects and is being used in treatment-resistant depression (TRD). Esketamine (Spravato®), a nasal spray formulation of the ketamine enantiomer, was approved by the FDA for TRD and Major Depressive Disorder (MDD) with acute suicidal ideation or behavior. Its ability to provide rapid relief, often within hours or days, contrasts with the typical weeks-long onset of most antidepressants, setting a high bar for emerging treatments.
2. Psilocybin Therapy
Research into psychedelics offers a novel approach to treating depression. Psilocybin, the active compound in "magic mushrooms," has demonstrated potential benefits in TRD and persistent depressive disorder in clinical trials. It's suggested to facilitate cognitive and emotional flexibility, allowing patients to break free from rigid negative thought patterns associated with depression. COMPASS Pathways is conducting phase 2b clinical trials of psilocybin therapy for TRD, highlighting its significance as an emerging competitor.
3. Rapid-Acting Antidepressants
Beyond ketamine, there is a push to develop other rapid-acting antidepressants that can provide relief within hours or days, rather than the typical several weeks. These include novel formulations and mechanisms of action that are currently under research and could significantly impact the treatment landscape if proven effective and safe.
4. Antidepressants with Novel Mechanisms
- Glutamatergic System: Drugs targeting the glutamatergic system, which is implicated in synaptic plasticity and depression, are under development.
- Inflammatory Pathways: Anti-inflammatory drugs are being investigated for depression linked to high levels of inflammation.
- Genetic and Biomarker-based Therapies: Treatments tailored to individuals' genetic makeup or specific biomarkers represent a frontier in personalized medicine for depression.
5. Digital and Technological Interventions
Digital therapeutics and tools, including apps and web-based platforms offering cognitive-behavioral therapy (CBT) and other psychological interventions, are gaining traction as adjunct or primary treatments for mild to moderate depression. While not pharmaceuticals, their growing acceptance and evidence base make them relevant competitors in the broader depression care ecosystem.
Competitive Considerations for PIPE-307
For PIPE-307 to successfully compete in this diverse and evolving landscape, its development and positioning will need to clearly demonstrate advantages in areas such as efficacy, particularly for treatment-resistant populations, speed of onset, safety profile, and ease of use. Additionally, as the understanding of depression deepens, treatments that can address specific patient subgroups or offer personalized approaches based on genetic or biomarker profiles may become increasingly important. PIPE-307's unique action on the M1R receptor offers a distinct approach that could provide cognitive benefits alongside mood improvement, differentiating it from many current and emergent therapies if these advantages are clinically confirmed.
Depression, a complex psychiatric condition characterized by persistent sadness, lack of interest, and various physical symptoms, has seen the development of numerous pharmacological treatments over the years. The goal of these treatments is to mitigate symptoms and improve the quality of life for patients. This response will highlight some of the most notable antidepressants, including those that have been recently approved, providing a snapshot of the evolving landscape of depression treatment.
Traditional Antidepressants
- Selective Serotonin Reuptake Inhibitors (SSRIs): Examples include sertraline (Zoloft), fluoxetine (Prozac), and citalopram (Celexa). SSRIs are often first-line treatments due to their relatively favorable side effect profiles.
- Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs): Venlafaxine (Effexor) and duloxetine (Cymbalta) are examples of this class, used for major depressive disorder (MDD), anxiety disorders, and chronic pain.
- Tricyclic Antidepressants (TCAs): Such as amitriptyline and nortriptyline. While effective, their use has declined due to more significant side effects compared to newer agents.
- Monoamine Oxidase Inhibitors (MAOIs): Such as phenelzine (Nardil). MAOIs are effective but often reserved for cases that do not respond to other treatments due to dietary restrictions and side effect concerns.
Recently Approved Drugs for Depression
- Esketamine (Spravato): Approved by the FDA in 2019 for treatment-resistant depression (TRD) and depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior. Administered as a nasal spray, it's a fast-acting treatment that belongs to a new class of drugs acting as NMDA receptor antagonists. Esketamine's approval marked a significant advancement in treating TRD, offering hope to patients who have not responded to standard treatments.
- Vilazodone (Viibryd) and Vortioxetine (Trintellix): Though not brand new, these drugs represent newer classes of antidepressants approved in the early 2010s. Viibryd was approved in 2011, and Trintellix in 2013 (initially approved as Brintellix). Both are used to treat major depressive disorder (MDD) and touted for their novel mechanisms of action and reduced sexual side effects, a common issue with many traditional antidepressants.
- Brexanolone (Zulresso): Approved in 2019, this is the first medication specifically indicated for postpartum depression. Administered via continuous IV infusion over 60 hours, it's derived from allopregnanolone, a naturally occurring steroid in the brain that modulates the GABA system.
Preclinical data
Depression
The preclinical data outlines the development and evaluation of PIPE-307, a novel small molecule that selectively inhibits the M1 muscarinic receptor (M1R), for its potential application in treating depression and relapsing-remitting multiple sclerosis (RRMS). Here's a summary of the key findings:
-
Toxicity and Safety Pharmacology
- The safety profile of PIPE-307 was established through comprehensive nonclinical studies involving rodents and dogs. These included pivotal toxicology studies with daily oral dosing for up to six months in rodents and nine months in dogs, with recovery phases as needed.
- The safety pharmacology studies, conducted according to Good Laboratory Practice (GLP) standards, assessed cardiovascular, respiratory, and central nervous system (CNS) functions in rodents and dogs, alongside embryo-fetal development studies in rodents and rabbits.
-
In Vitro Activity and Selectivity
- PIPE-307 demonstrated potent inhibition of acetylcholine-induced Ca2+ mobilization in cells overexpressing M1R, with an IC50 value of 3.8 nM. Notably, it showed more than 25-fold functional selectivity over other muscarinic isoforms (M2 through M5), underscoring its targeted action on M1R.
-
In Vivo M1R Occupancy Studies
- Dose-dependent M1R occupancy in the brain was confirmed using [3H]-PIPE-307 as a radiotracer, with an ED50 of 0.4 mg/kg. Consistent plasma and brain EC50 values of 96 nM were reported, with a corrected unbound plasma EC50 of 9 nM considering 91.2% rodent plasma protein binding.
- Time-course studies revealed sustained M1R occupancy levels: over 60% for at least 8 hours at 3 mg/kg, and over 90% for the same duration at 30 mg/kg. Occupancy declined over time but maintained at least 50% for approximately 16 hours at higher doses.
-
Preclinical Proof-of-Concept for Depression Treatment
- PIPE-307 binds with high affinity to M1R and selectively compared to other muscarinic receptors. Preclinical studies showed increased synaptic transmission and plasticity in the medial prefrontal cortex (mPFC), including enhanced mini excitatory postsynaptic current (mEPSC) amplitude and increased presynaptic release events 24 hours after dosage.
- In vivo, PIPE-307 demonstrated antidepressant-like effects in the Porsolt swim test (PST) in rodents, both in single-dose and repeated daily dosing paradigms. A single oral dose significantly reduced immobility time in a dose-dependent manner, indicating antidepressant activity. Repeated daily administration for seven days showed comparable efficacy at 30 mg/kg/day to a single dose, with improved efficacy at 3 mg/kg/day to a level similar to the 30 mg/kg/day dose.
In summary, the preclinical data for PIPE-307 indicates a promising safety profile and potent, selective inhibition of M1R, with significant potential for treating depression and RRMS. The molecule's ability to induce targeted receptor occupancy and enhance synaptic plasticity and transmission supports its further development and clinical evaluation in these therapeutic areas.
The preclinical findings for PIPE-307 as a selective inhibitor of the M1 muscarinic receptor (M1R) for the potential treatment of depression and relapsing-remitting multiple sclerosis (RRMS) are significant on multiple fronts, particularly in terms of their potential translatability to human conditions. Here's an analysis based on the current scientific and clinical literature:
-
Significance of Findings
- High Selectivity and Potency: The demonstrated selectivity of PIPE-307 for M1R over other muscarinic receptors is particularly significant. M1 receptors play a crucial role in cognitive function and are implicated in the pathophysiology of both depression and neurodegenerative diseases like RRMS. The high potency (IC50 of 3.8 nM) indicates that PIPE-307 can effectively inhibit M1R at low concentrations, which may minimize side effects associated with broader muscarinic activity.
- Safety Profile: The comprehensive safety pharmacology and toxicology studies, especially with long-term dosing in rodents and dogs, are foundational for progressing to human trials. The absence of significant adverse findings in these studies supports the potential for a favorable safety profile in humans.
- In Vivo Efficacy: Demonstrated dose-dependent receptor occupancy and sustained engagement over time suggest that PIPE-307 has a pharmacokinetic profile that could support once or twice daily dosing in humans, which is crucial for patient compliance and therapeutic efficacy.
-
Predictive Validity and Translatability
- Disease Models: The use of in vivo models to demonstrate antidepressant-like effects, particularly through the Porsolt swim test (PST), offers a measure of predictive validity. However, while PST is widely used for screening antidepressants, its translatability to human depression is debated due to differences in the complexity of human depression versus stress-induced behaviors in rodents. Similarly, for RRMS, the absence of specific data on models like experimental autoimmune encephalomyelitis (EAE) limits direct translatability to human disease, as EAE is a common model that mimics aspects of MS pathology.
- Synaptic Plasticity Measures: The observed effects on synaptic transmission and plasticity, such as enhanced mEPSC amplitude and presynaptic release events, align with the hypothesis that improving synaptic function could counteract the synaptic deficits observed in depression and neurodegenerative diseases. These findings have a solid basis in the understanding of disease pathophysiology and offer a mechanistic rationale for PIPE-307's therapeutic potential.
-
Considerations on Model Systems and Human Biology
- Species Differences: The primary concern with translating findings from rodent models to humans is the difference in physiology and disease pathology. For example, the distribution and function of muscarinic receptors may vary between species, potentially affecting drug efficacy and safety profiles.
- Complexity of Human Diseases: Diseases like depression and RRMS are multifactorial, with genetic, environmental, and neurobiological contributors. This complexity is difficult to fully recapitulate in animal models, which may focus on specific disease aspects or mechanisms.
- Biomarkers and Endpoints: The translation of preclinical efficacy to clinical settings may also be challenged by the lack of direct biomarkers or endpoints. For instance, changes in synaptic plasticity markers or receptor occupancy levels that are measurable in animal studies may not directly correlate with clinical outcomes in humans without validated biomarkers.
In conclusion, while the preclinical data for PIPE-307 is promising and supports further development, the transition from preclinical to clinical stages will require careful consideration of these factors. It will be crucial to design early clinical trials that can validate the predictive markers of efficacy, optimize dosing, and assess safety in a way that accounts for the complexities of human biology and disease pathology.
Clinical trial overview
Summary of PIPE-307 Study Design
The PIPE-307 study is a Phase 1, single-center, open-label, adaptive-design PET study aimed to investigate the occupancy of brain muscarinic Type 1 receptors (M1AChR) by PIPE-307 in healthy volunteers. The study uses the radioligand [11C] PIPE-307 to determine this relationship. It is categorized as interventional with the primary purpose of treatment, focusing on single group assignment without any form of masking (open-label).
Key elements of the study include:
- Condition Investigated: Multiple Sclerosis.
- Intervention: A single oral dose of PIPE-307, followed by an intravenous dose of the radioligand [11C] PIPE-307 for PET imaging.
- Enrollment: 6 participants.
- Design: Adaptive design, evaluating the relationship between PIPE-307 exposure and brain M1AChR occupancy.
Critiques of the Study Design
Strengths
- Adaptive Design: Allows flexibility in study protocol based on interim data. This can lead to more efficient and potentially faster study outcomes.
- PET Imaging: Use of PET imaging provides a direct and quantitative measure of M1AChR occupancy, allowing for precise pharmacodynamic assessments.
Weaknesses and Challenges
- Small Sample Size: The enrollment of only 6 participants might limit the statistical power of the study and its ability to generalize findings.
- Healthy Volunteers: Testing in healthy volunteers rather than individuals with multiple sclerosis or depression may not accurately reflect the drug's effects in the target patient population.
- Open-Label Design: Without masking, there could be biases in assessment and participant behavior, potentially affecting study outcomes.
- Single-Center Study: Results from a single center may not be widely applicable, reducing the external validity of the findings.
Operational or Technical Challenges
- Recruitment and Retention: With a highly specific and small population, recruiting and retaining participants can be challenging.
- Technical Complexity: The use of [11C] PIPE-307 and PET imaging requires specialized equipment and expertise, limiting the study to facilities capable of conducting such work.
- Radioligand Production: The synthesis of [11C] labeled compounds is technically sophisticated, requiring on-site or nearby cyclotron facilities due to the short half-life of carbon-11 (~20 minutes).
- Data Analysis: Adaptive design necessitates robust data monitoring and analysis frameworks to inform study adjustments, demanding significant statistical and methodological expertise.
- Safety Monitoring: Given the study involves an investigational drug and radioligand, a rigorous monitoring plan for adverse effects is essential, particularly since the study involves healthy volunteers.
Conclusion
The PIPE-307 study employs an innovative approach to understand the pharmacodynamics of PIPE-307 in the context of M1AChR occupancy. While it incorporates several methodological strengths, the design and operational challenges such as small sample size, recruitment, technical complexity, and the need for extensive safety monitoring must be carefully managed to ensure the validity and applicability of the study results.
The PIPE-307 study, being a Phase 1 trial designed predominantly to evaluate the safety and pharmacokinetics of PIPE-307, also aims to provide early insights into the pharmacodynamic effects of PIPE-307 on brain M1AChR occupancy in healthy volunteers. While the direct applicability of the findings to specific diseases like RRMS and depression may be limited at this stage, establishing the link between PIPE-307 plasma concentrations and M1AChR occupancy can serve as a foundational proof-of-concept for further investigation in targeted patient populations.
Appropriateness of Primary and Secondary Endpoints
- Primary Endpoint: M1AChR receptor occupancy as determined by regional total volume of distribution (VT) of [11C] PIPE-307 at 24 hours post-dose is highly appropriate as a primary endpoint for this study. It directly measures the intended biological effect of PIPE-307, linking the pharmacological action of the drug to its potential therapeutic effects.
- Secondary Endpoints: The documentation does not list specific secondary outcomes, which is a missed opportunity to explore additional pharmacodynamic effects, safety profiles, or preliminary efficacy signals that could inform future studies in RRMS and depression.
Inclusion/Exclusion Criteria and Their Impact on Reproducibility and Applicability
- Inclusion Criteria: Restricting the study to normotensive males and females aged 25-65 who are deemed healthy based on clinical history and tests ensures a controlled environment to study the pharmacodynamic properties of PIPE-307 without interference from comorbidities or medications. However, this may limit the generalizability of the findings to the patient populations of interest, as individuals with RRMS or depression may have different physiological and metabolic profiles.
- Exclusion Criteria: The criteria aiming to exclude individuals with clinically relevant abnormalities, acute or chronic illnesses, and contraindications to study procedures are standard and help to ensure participant safety. However, the exclusion of individuals with substance abuse issues, smokers of more than 10 cigarettes daily, and those with significant research-related radiation exposure may exclude a subset of patients with depression or RRMS, potentially impacting the reproducibility and applicability of the study findings to these groups.
Potential Reproducibility Challenges
- Population Homogeneity: Conducting the study in a highly selected group of healthy volunteers may challenge the reproducibility of results across more diverse and clinically relevant populations, including those with RRMS or depression.
- Technical and Operational: The technical complexity of PET imaging with [11C] PIPE-307 and the necessity for specialized equipment may limit the number of sites able to reproduce the study findings accurately.
- Radiation Exposure Limits: The exclusion criterion related to previous research-related radiation exposure could limit the pool of potential participants, especially in populations already undergoing regular imaging studies for their condition, complicating reproducibility across studies.
While the PIPE-307 study is well-positioned to provide early proof-of-concept regarding the pharmacodynamics of PIPE-307 in healthy volunteers, challenges remain in directly translating these findings to therapeutic potential in RRMS and depression. Future studies should aim to include patient populations directly affected by these conditions to assess clinical efficacy and safety more appropriately. Adjusting inclusion and exclusion criteria to better match the demographic and clinical characteristics of these patient populations would enhance the clinical relevance and applicability of the research findings.
Clinical trial data
The Phase 1 PET trial for PIPE-307 was designed to assess brain receptor occupancy in healthy volunteers using PET imaging after a single oral dose of the drug. The trial had two main objectives:
- Primary Objective: To determine the M1 acetylcholine receptor (M1AChR) occupancy in the brain using [11C] PIPE-307 PET imaging after a single oral dose.
- Secondary Objective: To determine the relationship between the plasma concentration of PIPE-307 and the time-course of M1AChR occupancy, also using [11C] PIPE-307 PET imaging, following a single oral dose.
The study met both primary and secondary objectives and included three dose cohorts, with two subjects in each, at 10, 20, and 40 mg. Importantly, no safety concerns were noted with the single doses administered.
Key findings from the PET imaging study are as follows:
- Receptor Occupancy: A sigmoidal dose-response curve was observed, indicating that M1 receptor occupancy in the brain increased with plasma concentration of PIPE-307.
- EC50 Value: The effective concentration of PIPE-307 needed to achieve 50% of maximum receptor occupancy was established at 28.8 ng/mL.
- 95% Confidence Interval: The EC50 value's confidence interval ranged between 21.3 and 36.2 ng/mL, giving a statistical range where the true EC50 is likely to lie.
- Dose Range for Daily Administration: Based on the PET kinetics and receptor occupancy data, a daily dose range of 10 to 20 mg of PIPE-307 is suggested to be effective.
- Receptor Occupancy Plateau: The graph indicated that receptor occupancy reaches a plateau, meaning there is a maximum level of receptor occupancy PIPE-307 can achieve in the brain, beyond which increased concentrations do not result in higher occupancy.
- Variability: There was some variability in individual responses to the drug at different concentrations, which is common in pharmacological studies.
In conclusion, the Phase 1 PET trial's results for PIPE-307 show a quantifiable and predictable brain receptor occupancy with no associated safety concerns, supporting the dosage range of 10 to 20 mg for further clinical development. The receptor occupancy profile is critical for optimizing the dosing regimen to achieve the intended pharmacological effect.
Clinical trial overview
Summary of Study Design
PIPE-307's Phase 1 study focuses on assessing the safety, tolerability, and pharmacokinetics of a drug intended for the treatment of multiple sclerosis, specifically in healthy volunteers. This Phase I trial is divided into three parts:
- Single Ascending Dose (SAD) Study (Part 1): A cohort of approximately 48 subjects will be enrolled for a 6-week duration. In this part, different doses of PIPE-307 or a placebo will be administered to successive groups of subjects to evaluate the safety and pharmacokinetics of escalating single doses.
- Multiple Ascending Dose (MAD) Study (Part 2): This part involves approximately 24 subjects across a 7-week period, where subjects receive multiple doses of the drug or placebo. The aim is to assess the safety and pharmacokinetics when administered repeatedly over time.
- Food Effect Study (Part 3): A selected cohort from Part 1 will proceed to this 6-week study, which investigates the impact of food on the bioavailability of PIPE-307. Around 8 subjects will participate in this section.
The study is a randomized, double-blind, placebo-controlled trial involving sequential assignment of participants to either the experimental drug (PIPE-307) group or a placebo comparator group. Safety assessments will include monitoring of vital signs, physical exams, electrocardiograms, blood tests, and the recording of adverse events (AE).
Study Critique and Challenges
Critiques:
- Population Representativeness: By focusing on healthy volunteers, the study may not fully account for the physiological or metabolic differences seen in the patient population (those with multiple sclerosis or depression), which could affect the drug's pharmacokinetics, safety, and efficacy.
- Duration of Follow-up: The follow-up period (7 days post-dosing for SAD and 21 days for MAD) may not be sufficient to capture all delayed adverse effects or to fully understand the drug's pharmacodynamics and long-term safety profile.
- Food Effect Study Cohort Size: With only about 8 subjects in Part 3, the sample size may be too small to draw definitive conclusions about the impact of food on drug bioavailability, potentially limiting the generalizability of the findings.
Operational and Technical Challenges:
- Subject Compliance: Ensuring compliance with the study protocol, especially in outpatient settings, can be challenging. This includes adherence to dosing schedules and dietary restrictions.
- Placebo Effect: In double-blind, placebo-controlled trials, especially those involving subjective endpoints (e.g., reporting side effects), differentiating the true pharmacological effects from placebo responses can be difficult.
- Recruitment and Retention: Given the extensive commitments required from participants (multiple visits, adherence to strict study protocols), recruiting and retaining a sufficient number of eligible subjects may pose challenges.
- Safety Monitoring: The escalated dosing regimen in both the SAD and MAD phases requires rigorous safety monitoring to promptly identify and address any adverse effects, necessitating robust operational infrastructure and close monitoring.
In summary, while the PIPE-307 study is meticulously designed to evaluate the initial safety and pharmacokinetics of a potential treatment for multiple sclerosis, certain aspects such as its representativeness of the target patient population, the brief follow-up period, and operational complexities related to participant management could potentially impact the study's outcomes and generalizability.
Potential for Proof-of-Concept in Relapsing-Remitting Multiple Sclerosis and Depression
The Phase I study of PIPE-307 primarily aims to evaluate the safety, tolerability, and pharmacokinetics of escalating doses in healthy volunteers, which is a critical first step in drug development. However, to assess the potential of PIPE-307 as a treatment for relapsing-remitting multiple sclerosis (RRMS) and depression, it's important to understand the limitations and potentials of this early-phase research.
Appropriateness of Primary and Secondary Endpoints
- Safety and Tolerability (Primary Endpoint): These endpoints are appropriate for a Phase I study where the immediate goal is to ensure that PIPE-307 can be safely administered to humans. Information on treatment-emergent adverse events (TEAEs) is crucial before proceeding to disease-specific cohorts. However, safety alone does not provide evidence of efficacy in RRMS or depression.
- Pharmacokinetics and Pharmacodynamics (Secondary Endpoints): Understanding the drug's absorption, distribution, metabolism, and excretion (PK) and its mechanism of action (PD) are foundational. However, for a proof-of-concept regarding RRMS and depression, further studies focusing on disease-specific biomarkers, clinical outcomes, and symptomatology improvement are needed.
Inclusion / Exclusion Criteria Challenges
The inclusion criteria (normotensive males and females aged 25-65, deemed healthy) and exclusion criteria (lack of medical conditions, no drug or heavy alcohol use, and others) aim to ensure a homogeneous, healthy study population to reduce variability in safety and PK/PD responses. While this is appropriate for initial safety assessments, these criteria also pose several challenges:
- Generalization to Patient Populations: The strict health requirements exclude individuals with RRMS, depression, or comorbid conditions, which could limit the generalizability of study findings to actual patient populations. Patients with RRMS or depression often have comorbidities or take other medications that could influence the efficacy and safety profile of PIPE-307.
- Reproducibility Challenges: The strict criteria may also challenge reproducibility. In real-world settings, patients often diverge from the "ideal" profile of clinical trial participants. Factors such as mild comorbidities, previous or concurrent medication use, and broader age ranges can affect drug efficacy and safety, potentially leading to different outcomes in later-phase trials or post-marketing.
Clinical data
The Phase 1 study of PIPE-307 in healthy volunteers was a randomized, double-blind, placebo-controlled trial that evaluated the safety, tolerability, and pharmacokinetics (PK) of both single ascending doses (SAD) up to 80 mg and multiple ascending doses (MAD) up to 20 mg once daily for seven days. The study successfully completed all planned cohorts without any discontinuations and met its primary and secondary objectives.
- Safety and Tolerability: PIPE-307 was found to be safe and well-tolerated at all dose levels in both the SAD and MAD portions of the trial. There were no dose-limiting adverse events (AEs) or toxicities, and no serious or severe AEs reported. Treatment-emergent AEs (TEAEs) were generally mild and transient, with no clinically significant effects on safety laboratory tests, vital signs, or ECG.
- Effect of Food: There was no significant difference in the AE profile of PIPE-307 between fasted and fed conditions, indicating that food intake does not significantly alter the drug’s safety profile.
- Cognitive Function: No negative effects on higher cognitive functions, including psychomotor function, attention, memory, and executive function, were observed at key PK time points during the trial.
- Pharmacokinetics:
- Cmax: A dose-dependent increase in peak plasma concentration was observed. The 5 mg dose reached a Cmax just above 25 ng/mL, the 10 mg dose reached a Cmax near 50 ng/mL, and the 20 mg dose approached 100 ng/mL.
- Half-life: The half-life appeared to be consistent across doses, suggesting linear pharmacokinetics with regard to elimination.
- Dose Response: There was a direct relationship between dose and overall exposure to the drug, with both Cmax and AUC increasing with dose.
- Tmax: The time to reach Cmax was similar across all doses, occurring approximately within the first 2 hours post-administration.
- Elimination Phase: The drug concentration decreased over time at a rate that did not vary significantly with dose.
- Variability: There was some variability among subjects, particularly at higher concentrations, which is typical in early-stage clinical trials.
Overall, the results of this Phase 1 trial for PIPE-307 in healthy volunteers suggest that the drug is safe and has a predictable PK profile, with no observed negative impact on cognitive functions.
Clinical trial overview
The Phase 2 study is an interventional, randomized, double-blind, placebo-controlled, multicenter, dose-ranging study. It aims to evaluate the safety and efficacy of the drug PIPE-307 as an adjunctive treatment for subjects with Relapsing-Remitting Multiple Sclerosis (RRMS). The study involves 168 participants who are divided equally into three groups (1:1:1) to receive either a low dose of PIPE-307 (Dose A), a high dose of PIPE-307 (Dose B), or a placebo. This division creates a parallel assignment intervention model. The study spans over approximately 30 weeks, with a 28-day screening period followed by a 26-week treatment period. The primary outcome measures include the number of participants with treatment-emergent adverse events (TEAEs) and changes in binocular 2.5% low contrast letter acuity (LCLA) from baseline to the end of the treatment period. Secondary outcomes include the percentage of subjects with a certain level of improvement in various clinical and MRI measures of disease activity and progression.
Critique of the Study Design
- Randomization and Blinding: The study design involves randomized allocation and triple blinding (participant, care provider, investigator), which are strong points that minimize bias and provide a solid basis for comparison between the drug's effectiveness and its safety profile compared to placebo.
- Cohort Size: With 168 subjects and a 1:1:1 allocation ratio, each group will have relatively small sample sizes, which may affect the statistical power of the study to detect significant differences, especially for secondary outcomes or subgroup analyses.
- Outcome Measures: The selection of both safety and efficacy measures, including clinical assessments (e.g., T25WT, 9HPT, SDMT) and biomarkers (e.g., serum NfL), is comprehensive. However, the reliance on LCLA as a primary efficacy endpoint should be justified since it measures a very specific aspect of visual function that may not fully capture broader neurological changes in RRMS.
- Treatment Duration: The 26-week treatment period is relatively short for a disease-modifying therapy in RRMS, which may limit observations of long-term efficacy and safety.
- Pharmacokinetics: The study includes pharmacokinetic assessments, which are crucial for understanding the drug's metabolism and systemic exposure over time. However, the brief description does not specify the timing of these assessments beyond mentioning a measurement at week 30, which may miss critical early or peak concentrations.
Operational or Technical Challenges
- Patient Recruitment and Retention: Recruiting subjects with RRMS who meet the study criteria may be challenging, and maintaining their participation over the 30-week period requires careful planning and support, especially in a multicenter setting.
- Blinding Integrity: Ensuring the placebo and PIPE-307 doses are indistinguishable in appearance, taste, and packaging is crucial for maintaining the study's double-blind integrity. This requires meticulous preparation and monitoring.
- Data Collection and Management: Accurately collecting, managing, and analyzing data from various sources, including clinical assessments, MRI, and blood samples, across multiple centers, necessitates a robust data management system and rigorous quality control processes.
- Regulatory and Ethical Considerations: Conducting a study on a drug for a condition like RRMS involves navigating complex regulatory requirements and ethical considerations, especially regarding participant safety, informed consent, and reporting adverse events.
- COVID-19 Impact: Given the ongoing global situation regarding COVID-19 (as of the knowledge cut-off in 2023), there might be unforeseen challenges related to site access, participant safety, and study timelines.
In summary, while the design of the PIPE-307 study incorporates several strong elements for assessing the drug's safety and efficacy, there are inherent challenges and considerations that need careful management to ensure the reliability and applicability of the study outcomes.
The potential of this study to provide proof-of-concept for the use of PIPE-307 in relapsing-remitting multiple sclerosis (RRMS) is grounded on its study design, including the selection of primary and secondary endpoints, as well as the clearly defined inclusion and exclusion criteria. There's no mention of depression in the eligibility or study design sections, suggesting a focus solely on RRMS, which should be made clear when discussing potential outcomes.
Appropriateness of Primary and Secondary Endpoints
- Primary Endpoints: The primary outcomes, particularly the measurement of treatment-emergent adverse events (TEAEs) and changes in binocular 2.5% low contrast letter acuity (LCLA), are appropriate for evaluating the safety profile of PIPE-307 and its potential impact on visual impairment, a common symptom in RRMS patients. LCLA specifically targets an aspect of neurological function relevant to the patient's quality of life, correlating with disease activity.
- Secondary Endpoints: The chosen secondary outcomes, including improvements in LCLA, functional disability assessments (Timed 25-Foot Walk Test, Nine-Hole Peg Test, Symbol Digital Modality Test), MRI measures of myelination and MS disease activity, and changes in serum neurofilament light chain, are comprehensive. They collectively aim to capture various dimensions of the disease's impact and the drug's potential multifaceted benefits.
Inclusion / Exclusion Criteria
The inclusion criteria are tailored to ensure that participants have a clear diagnosis of RRMS without significant comorbid conditions that could confound the effects of the intervention. Requirements for stable treatment regimens and other health parameters ensure a uniform study population, enhancing the interpretability of the results.
The exclusion criteria carefully screen out potential participants with recent MS exacerbations, other significant health issues, or treatments that could interfere with the study's objectives or affect the safety of the participants. These considerations are crucial for minimizing risk and ensuring that changes observed during the study can be attributed with greater confidence to PIPE-307.
Potential Reproducibility Challenges
- Narrow Age Range and Health Status: The strict age range (18 to 50 years) and requirement for overall good medical health, aside from RRMS, might limit the generalizability of the study findings to older patients or those with more complex health profiles.
- Specific Treatment Backgrounds: The requirement for participants to have been on no more than a single disease-modifying therapy (DMT) for RRMS and stable on this treatment for at least 6 months may exclude a significant portion of the RRMS population, particularly those with more aggressive disease or those who have switched DMTs due to ineffectiveness or side effects.
- CYP3A4 Interacting Substances: The exclusion of participants based on the use of substances that interact with the CYP3A4 enzyme could exclude patients on common treatments for comorbid conditions, potentially challenging the reproducibility of the study results across the broader RRMS population.
In conclusion, while the study design, including its endpoints and eligibility criteria, appears well thought out to provide a robust evaluation of PIPE-307's efficacy and safety in RRMS, the narrowness of the inclusion criteria and exclusion of certain populations may limit the application of the findings to the general RRMS population. Future studies may need to consider broader participant profiles to fully assess the drug's potential applicability.
Weekly analyses of biotech startups, generated by AI
Receive high quality, AI-generated analyses of biotech startups, public companies and scientific papers each week.