EyeBio investment analysis
November 14, 2023
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Update: In February 2024, EyeBio announced 12-week outcomes from its Phase 1/2 study. Read the analysis the data here.
Overview
EyeBio is a private biotech company developing treatments for retinal diseases. Their lead product, Restoret, is designed to activate the Wnt signaling pathway, believed to be crucial for regulating vascular integrity within the retina.
The company is studying Restoret in the AMARONE Phase 1/2 trial in Diabetic Macular Edema (DME) and Neovascular Age-Related Macular Degeneration (NVAMD) (also known as wet AMD).
The company expanded its Series A to $130 million in November 2023, with participation from Bain Capital Life Sciences, Omega Funds and Vertex Ventures HC, as well as existing investors SV Health Investors, Jeito Capital, Samsara Biocapital and MRL Ventures Fund.
Pipeline overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| Restoret | Tri-specific antibody | FZD4, LRP5 binder | Diabetic Macular Edema | |||||||
| Restoret | Tri-specific antibody | FZD4, LRP5 binder | Neovascular Age-related Macular Degeneration |
Highlights and risks
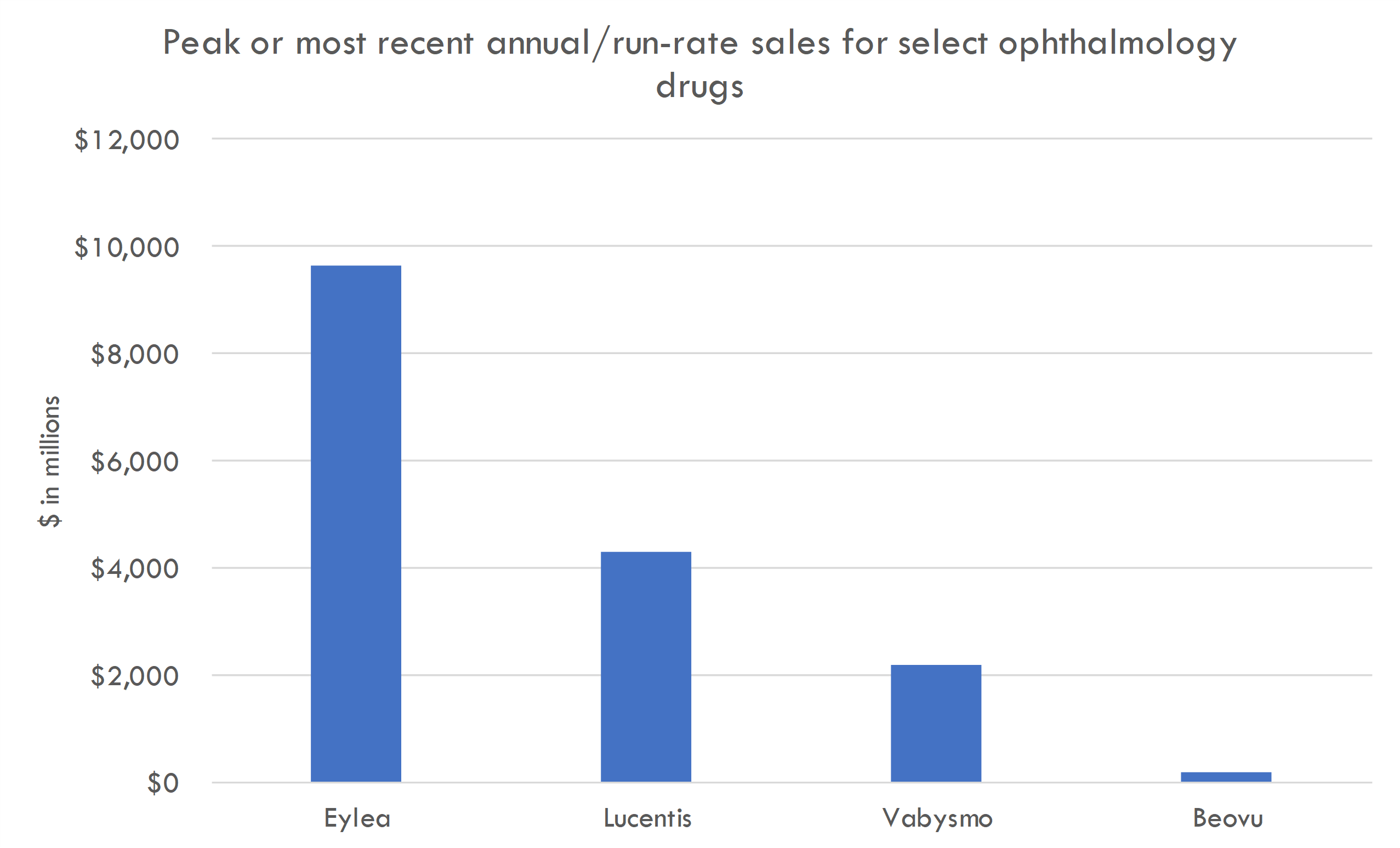
Targeting very large markets in DME and wet AMD. Approved drugs like Eylea generate several billion in peak sales in these indications.
Strong evidence supporting role of Wnt pathway in disease pathology.
Ongoing Phase 1/2 study will provide early proof-of-concept, although study is too small to understand commercial potential.
While scientific rationale is strong, pursuing a novel mechanism of action is risky.
Highly competitive space with both effective approved drugs and many development-stage drugs.
No clinical data directly supporting therapeutic hypothesis.
Valuation
Given the early stage of the company and limited information about its programs, we did not conduct a valuation analysis.
Scientific thesis
Diabetic macular edema (DME) and neovascular age-related macular degeneration (NVAMD) are prominent eye conditions that lead to vision loss and they share a common pathology characterized by breakdown of the blood-retinal barrier (BRB) and subsequent vascular leakage. The leakage from damaged microvasculature into the retinal tissue results in edema and progressive degeneration, ultimately impairing vision.
In patients with these conditions, the currently available therapies, particularly anti-vascular endothelial growth factor (anti-VEGF) treatments, have limitations. While effective for many, they do not consistently provide significant visual gains for all treated patients or long-term suppression of disease progression, as evidenced by the continued leakage and subsequent edema despite frequent and repeated injections.
Wnt signaling is a fundamental pathway involved in the regulation of vascular stability and integrity in the retina. The tri-specific antibody Restoret, under development by EyeBio, specifically targets FZD4 and LRP5, which are core receptors in the Wnt pathway regulating vascular integrity in the retina. Restoret is a Norrin mimetic, meaning that it mimics the function of Norrin—a natural ligand essential for the function of the Wnt pathway in retinal vascular health. By binding to FZD4 and LRP5, Restoret aims to activate the downstream beta-catenin signaling pathway, which is responsible for the upregulation of tight junction proteins that maintain the BRB.
The therapeutic rationale behind such an approach draws from the understanding that by acting on the Wnt signaling pathway, one can stimulate the endogenous mechanisms that maintain and repair the blood-retinal barrier, thereby potentially resolving the leakage irrespective of the driving cause, which could be either increased VEGF levels in DME and NVAMD or mutations in components of the Norrin/Wnt pathway as seen in diseases such as familial exudative vitreoretinopathy (FEVR).
Restoret is engineered to have high specificity and affinity for its targets, which confers several potential advantages:
Restoration and Maintenance of BRB: By activating the Wnt pathway, Restoret promotes the restoration of paracellular tight junctions between endothelial cells, reducing vascular permeability and leakage.
Durability: Due to its potential to reactivate the body's own repair mechanisms, there may be a longer-lasting benefit compared to existing treatments that simply block leakage factors like VEGF.
Broad Applicability: Its mode of action suggests that Restoret may be effective in a wide range of retinal diseases that feature disruption of the BRB.
Discussion of evidence base
The science relating to the role of the Wnt signaling pathway in maintaining the blood-retinal barrier (BRB) and vascular stability in the retina is well-established. This pathway is known to be critical for the development of the retinal vasculature and the integrity of the endothelial cell junctions, which in turn prevents pathological fluid leakage. The scientific understanding of these processes is supported by decades of research, and dysregulation of Wnt signaling has been implicated in several retinal diseases.
The literature regarding the role of FZD4 and LRP5 in ocular diseases such as diabetic macular edema (DME) and neovascular age-related macular degeneration (NVAMD) is rooted in the understanding of the Wnt signaling pathway and its function in the vasculature of the retina.
FZD4 (Frizzled-4) and LRP5 (low-density lipoprotein receptor-related protein 5) are essential receptors in the canonical Wnt signaling pathway. The pathway's functionality is critical for the development and maintenance of the BRB, which, when compromised, is a significant contributor to the pathogenesis of DME and NVAMD.
Here are some key pieces of evidence from the literature:
Role of Wnt Signaling in Retinal Blood Vessel Development and Stability: The Wnt/β-catenin signaling pathway, where FZD4 and LRP5 play crucial roles, is involved in the development and maintenance of the retinal blood vessels. Disruption of this pathway often leads to retinal vascular diseases. (Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883-895.)
Familial Exudative Vitreoretinopathy (FEVR): Mutations in FZD4 and LRP5 are known to cause familial exudative vitreoretinopathy, a hereditary disorder that affects the development of the retinal vasculature, supporting their role in retinal vascular health. Although FEVR is a different condition, the involvement of the Wnt pathway suggests parallels to DME and NVAMD, as both these diseases involve similar vascular components. (Robitaille JM, Wallace K, Zheng B, et al. Phenotypic overlap of familial exudative vitreoretinopathy with persistent fetal vasculature and Coat's disease within one family. Ophthalmic Genet. 2009;30(1):23-30.)
Evidence in Animal Models: Studies in mice have shown that activation of the Wnt signaling pathway through Norrin, which interacts with FZD4 and LRP5, can protect against retinal vascular leakage and may be a potential therapeutic target for retinal diseases where the BRB is disrupted. (Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y, Zhou T, et al. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc Natl Acad Sci U S A. 2010;107(15):6900-6905.)
Wnt Pathway Activation for Therapeutic Benefit: Additional preclinical research has indicated that pharmacologic activation of the Wnt signaling pathway can lead to restoration of vascular integrity in the retina and could be a strategy for treating diseases characterized by vascular leakage, like DME and NVAMD. (Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J Biol Chem. 2007;282(6):4057-4068.)
Challenges in Anti-VEGF Treatment: Clinical practice and research have observed that despite the efficacy of anti-VEGF treatments in DME and NVAMD, a significant proportion of patients continue to experience disease progression or recurrence, leading researchers to explore alternative pathways like Wnt signaling for additional therapeutic strategies. (Sophie R, Hafiz G, Scott AW, Zimmer-Galler I, Nguyen QD, Ying H, et al. Long-term Outcomes in Ranibizumab-Treated Patients With Retinal Vein Occlusion; The RETAIN Study. Ophthalmology. 2015;122(1):153-160.)
The evidence base supporting the therapeutic rationale for using a Norrin mimetic tri-specific antibody that binds to FZD4 and LRP5 to treat retinal diseases like diabetic macular edema (DME) and neovascular age-related macular degeneration (NVAMD) consists primarily of basic science research, preclinical studies, and, increasingly, early-phase clinical trials. Here are the strengths and weaknesses of this evidence base:
Strengths:
Molecular and Cellular Biology Understanding: The Wnt signaling pathway's role in vascular development and maintenance is well-characterized in molecular and cellular biology, providing a strong foundation for targeting this pathway therapeutically.
Preclinical Models: Animal studies have shown that modulation of the Wnt pathway can impact vascular permeability and repair, suggesting that similar effects could be achieved in human retinal diseases.
Genetic Evidence: Observations in humans, such as mutations in FZD4 and LRP5 leading to FEVR, underline the importance of the Wnt pathway in retinal vascular health. This provides genetic support for targeting these receptors in retinal disease.
Biotechnology Advancements: Recent protein engineering advancements allow for the development of tri-specific antibodies that can precisely target components of the Wnt pathway with high affinity, increasing the specificity and potential efficacy of the therapy.
Anti-VEGF Limitations: There is clear clinical evidence that not all patients with DME and NVAMD respond adequately to anti-VEGF treatments, highlighting the need for alternative therapeutic options and supporting the therapeutic rationale for different targets.
Weaknesses:
Translation to Humans: Animal models do not always accurately predict human responses, and many potential therapeutics that showed promise in preclinical studies have failed in human trials.
Long-term Safety: Long-term effects of Wnt pathway activation are not yet fully understood. Chronic activation could lead to off-target effects or promote other pathologies, such as fibrosis or tumor growth, given the pathway's roles in cell proliferation and differentiation.
Complex Pathophysiology: Retinal diseases like DME and NVAMD have multifaceted etiologies. Targeting a single pathway may not address all of the significant drivers of the disease.
Clinical Trial Outcomes: The outcomes of clinical trials for Restoret are not yet known. The evidence supporting the therapeutic rationale will either be bolstered or weakened by the findings of these trials.
Comparative Efficacy: There's not yet evidence that Wnt pathway activators are superior or even equivalent to current standards of care in terms of efficacy or safety.
In conclusion, the research literature supports the exploration of Wnt signaling and the role of FZD4 and LRP5 as therapeutic targets in DME and NVAMD. However, it's worth highlighting that the majority of this evidence comes from basic scientific research and animal models. The translation of this knowledge into effective clinical treatments for humans is still underway, and the ongoing clinical trials for therapeutic candidates like Restoret are critical to establishing clinical applicability. The definitive evidence that supports the role of these receptors in the pathology of DME and NVAMD in human patients will be the successful demonstration of safety and efficacy through these trials.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
Clinical trial overview
Restoret is currently being studied in a Phase 1/2 study in DME and NVAMD / wet AMD.
Summary of the Study Design:
The study design for Restoret (EYE103-101) is constructed as a two-part clinical trial, purposed to evaluate the safety and preliminary efficacy of the drug EYE103 in patients with Diabetic Macular Edema (DME) as a monotherapy or in combination with anti-VEGF for Neovascular Age-related Macular Degeneration (NVAMD).
Part 1 – Multiple Ascending Dose (MAD):This open-label portion of the study will explore the safety profile of EYE103 at increasing dosage levels. Around 12 participants will be recruited, receiving sequentially higher doses to determine the highest dose tolerated without significant adverse effects. This section helps establish the safety threshold of the drug.
Part 2 – Dose Finding Study:In a single-masked format, around 80 participants will be randomized to receive one of two selected doses of EYE103 to assess and compare the drug's effectiveness. This part of the study will use a triple-masked approach (also known as double-masked plus one), with masking of the participants, care providers, and outcomes assessors to minimize bias.
Critiques of the Study Design:
1. Small Sample Size in Part 1:While typical for Phase 1 studies, the small sample size may limit the generalizability of the safety findings across a more diverse population.
2. Multiple Indications:Testing EYE103 both as a monotherapy for DME and in combination with NVAMD patients could introduce complexity in interpreting results if the drug behaves differently under these conditions. The study might have to contend with different mechanisms of disease and drug action in the two conditions.
3. Masking/Blinding:The first part is open-label, which may introduce observer bias. However, this is standard in a dose-escalation study where the primary concern is safety. The second part is adequately masked, but the operational implementation of successful masking must be ensured for validity.
Operational and Technical Challenges:
1. Enrollment and Retention:Recruiting enough suitable candidates for both conditions within a specified timeframe could be challenging. Ensuring participant retention over the study duration, especially during and after dose escalation, can be difficult if adverse effects arise.
2. Drug Administration and Monitoring:Intravitreal (IVT) administration requires precise technique and carries risks of intraocular infection or damage. Furthermore, frequent monitoring of participants for safety and efficacy endpoints will necessitate strict adherence to protocols and careful scheduling.
3. Data Management:Considering the study is split into two distinct parts with different participant populations and treatment modalities, efficient data handling systems must be in place to track and analyze these separate data streams accurately.
4. Outcome Assessment:Evaluating outcomes such as best-corrected visual acuity requires standardized testing conditions and skilled personnel to minimize variability in measurements.
The potential of this study (EYE103-101) to provide proof-of-concept for the use of Restoret in Diabetic Macular Edema (DME) during Phase 1/2 trials is contingent upon the appropriateness of the chosen endpoints and inclusion/exclusion criteria, as these will guide the validity and interpretability of the findings. Here's an analysis:
Appropriateness of Primary and Secondary Endpoints:
The primary endpoint of adverse events (AEs) is fundamental for a Phase 1/2 study, as it is primarily concerned with safety. For proof-of-concept, it is critical to demonstrate that Restoret can be administered at efficacious doses without unacceptable toxicity. The secondary endpoint of best-corrected visual acuity (BCVA) is also suitable, as it is directly relevant to the patient experience of the disease and is a commonly accepted clinical endpoint for DME trials.
Appropriateness of Inclusion/Exclusion Criteria:
- Age Criteria: The age distinction between DME (≥ 18 years) and NVAMD (≥ 50 years) aligns with the epidemiology of these conditions, as NVAMD typically affects older individuals.
- Diagnoses & Treatment History: This criterion that DME patients must be treatment-naïve ensures the study evaluates Restoret without interference from other treatments. Allowing NVAMD patients to be treatment-naïve or experienced reflects the reality of NVAMD management, where patients often undergo multiple therapies, hence testing Restoret in a more representative sample.
- Vision Loss Requirement: Including only those with vision loss in the study eye ensures that the study includes patients with clinically significant disease who may benefit from therapy and who can contribute relevant efficacy information.
Exclusion Criteria:
- Pregnancy & Breastfeeding: Excluding these populations is standard to prevent any drug-related harm to the fetus/baby, although it reduces the generalizability to all DME patients, considering DME can affect women of childbearing age.
- Recent Eye Surgeries: Ensuring the ocular structure is stable and has not been modified recently by other procedures is important to isolate the drug's effect accurately.
- Other Excluding Conditions: Ruling out individuals with other ocular conditions is crucial to attribute changes in vision to the study drug rather than unrelated ocular pathology.
Reproducibility Challenges Posed by Inclusion/Exclusion Criteria:
- Heterogeneity in Disease Severity: Given that patients may come in with varying levels of vision loss but are all considered treatment-naïve (for DME) or with variable treatment experience (for NVAMD), there may be differences in responses to treatment and disease progression that could affect reproducibility.
- Consistency in Diagnostic Criteria: Ensuring that the diagnosis of DME or NVAMD is made consistently across study sites could be challenging and affect reproducibility if not standardized.
- Differential Progression Rates: The natural course of disease varies among individuals; thus, capturing and controlling for this variation during the relatively short timeframe of a clinical trial is challenging, especially when considering a measure like BCVA.
To maximize the study's capacity to provide strong proof-of-concept evidence, it is crucial for the criteria for inclusion to be applied consistently across individuals and for the endpoints to be measured with rigorous, standardized methods. This will help ensure that the results are reliable and can be reproduced in a larger, more diverse patient population in subsequent trials.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
Market overview
Diabetic Macular Edema
Diabetic Macular Edema (DME) is a significant cause of visual impairment among individuals with diabetes, characterized by the accumulation of fluid in the macula due to leaking blood vessels. It is a complication of diabetic retinopathy. As the global prevalence of diabetes increases, so does the market opportunity for DME treatments.
Successful drugs for DME:Several drugs have been successfully developed and marketed for the treatment of DME, largely belonging to the class of anti-VEGF (vascular endothelial growth factor) agents, which help reduce vascular leakage and inflammation. Some of the notable drugs include:
- Ranibizumab (Lucentis) – Developed by Genentech/Roche, this was the first anti-VEGF specifically approved for DME. Its success in the market set a benchmark for other anti-VEGFs.
- Aflibercept (Eylea) – Developed by Regeneron Pharmaceuticals, it has shown effectiveness in improving visual acuity in DME patients and has captured a significant market share.
- Bevacizumab (Avastin) – Although not officially approved for DME, bevacizumab is often used off-label due to its similar mechanism of action and lower cost compared to Lucentis and Eylea.
Corticosteroids like dexamethasone (Ozurdex) and fluocinolone acetonide (Iluvien) have also been used for their anti-inflammatory properties, especially in patients who may not respond adequately to anti-VEGF therapy.
Standard of Care:The current standard of care (SoC) for DME includes:
- Anti-VEGF injections (ranibizumab, aflibercept, off-label bevacizumab)
- Laser photocoagulation therapy – especially for patients who do not respond to medical therapies.
- Corticosteroids (as intravitreal implants or injections) – Often considered in cases where anti-VEGF does not yield desired outcomes or for long-term treatment to reduce the frequency of injections.
Unmet Medical Need:Despite the availability of these treatments, there remains a substantial unmet medical need in the treatment of DME:
- Treatment Burden: Current anti-VEGF therapies require frequent intravitreal injections, which can be burdensome for patients, influence compliance, and carry a risk of adverse events like infection or retinal detachment.
- Variable Response: Not all patients respond to anti-VEGF treatments, and the effectiveness can vary greatly among individuals.
- Long-Term Efficacy: There's a need for treatments with long-lasting effects to reduce the frequency of treatments and improve patient compliance.
- Preventive Therapies: Therapies that could prevent the progression of diabetic retinopathy to DME are still lacking.
- Alternative Treatment Options: There is an opportunity for the development of drugs with different mechanisms of action from current treatments that could serve patients who are non-responsive or intolerant to anti-VEGF therapies.
In summary, the market opportunity in DME therapeutics is driven by a rising diabetic population, the burdensome nature of current treatments, and the persistent need for more diversified and long-acting therapeutic options to enhance patient outcomes and quality of life. Companies that can address these unmet needs with innovative treatments are likely to find significant opportunities in this space. Continued research into the pathophysiology of DME could also lead to the identification of novel therapeutic targets and the development of new drugs, further expanding the market space.
Several promising treatments are in development for Diabetic Macular Edema (DME), targeting different aspects of the disease pathogenesis beyond just anti-VEGF pathways. Here's a brief overview of some of the approaches being explored:
New anti-VEGF agents: Although the market currently has effective anti-VEGF treatments for DME, research continues to develop new agents or formulations that may offer longer-lasting effects, reduced injection frequency, or improved efficacy. These next-generation anti-VEGF therapies aim to optimize patient adherence and outcomes.
Tie2/Angiopoietin pathway: The Tie2/angiopoietin pathway is involved in the angiogenesis and vascular stability. Therapeutic agents targeting the Ang-2/Tie2 pathway seek to enhance vascular stability and reduce leakage, which might address some of the limitations of current anti-VEGF therapies. Faricimab is an example that inhibits both VEGF and Ang-2.
-
Integrin inhibitors: Integrins are cell adhesion molecules implicated in several cellular processes including inflammation, fibrosis, and vascular leakage. Drugs targeting integrins are being researched for their potential to reduce macular edema and improve vision.
Corticosteroid implants: There is ongoing research to improve the delivery and efficacy of corticosteroids in DME. Sustained-release implants are being developed to provide long-term release of medication to the affected area, minimizing the need for frequent injections.
Gene therapies: These are being investigated for their potential in DME to provide a long-term therapeutic effect after a single treatment. Gene therapies could deliver DNA sequences coding for anti-VEGF or other antiangiogenic factors directly into the retina.
Combination therapies: Some studies are evaluating the synergistic effects of using two or more drugs with different mechanisms of action. For instance, combining an anti-VEGF with a corticosteroid or with a new class of medications may improve efficacy and durability of the treatment.
Inflammatory pathway inhibitors: Since inflammation plays a significant role in DME, drugs targeting various inflammation pathways are being studied. This includes inhibitors of cytokines and chemokines involved in the inflammatory response of DME.
Protease inhibitors: Proteolytic enzymes like matrix metalloproteinases are involved in the breakdown of the extracellular matrix and blood-retina barrier disruption. Inhibitors of these enzymes are being evaluated for their potential to treat DME.
Topical and oral treatments: To reduce the burden of intraocular injections, researchers are exploring eye drops and oral medications that can reach the retina and have a therapeutic effect on DME.
Stem cell therapy: Investigational treatments using stem cells are also being explored with the aim of regenerating damaged retinal cells and restoring vision.
It's important to understand that many of these treatments are still under clinical investigation, ranging from early-stage research to Phase III clinical trials. The safety and efficacy of these treatments will be established upon successful completion of clinical trials and approval from regulatory bodies like the FDA. As research advances, these emerging therapies may offer improved outcomes for patients with DME, meeting the current unmet needs and potentially reshaping the standard of care in the future.
There are several branded drugs notably used for the treatment of Diabetic Macular Edema (DME), which have been approved by regulatory authorities such as the U.S. Food and Drug Administration (FDA). These drugs primarily include anti-VEGF agents, corticosteroids, and some that have combined mechanisms of action. Here is a list and description of some of the key branded drugs used in the treatment of DME:
- Ranibizumab (Lucentis) This is a monoclonal antibody fragment developed by Genentech that inhibits VEGF-A, a protein that plays a critical role in the formation of new blood vessels (angiogenesis) and the leakage of fluid into the retina. Ranibizumab was one of the first anti-VEGF therapies specifically approved for the treatment of DME.
- Aflibercept (Eylea) Developed by Regeneron Pharmaceuticals, aflibercept is a soluble decoy receptor that binds VEGF-A, VEGF-B, and placental growth factor (PlGF), which are involved in the formation and leakage of abnormal blood vessels. It has been shown to be effective in improving vision in patients with DME.
- Bevacizumab (Avastin) Although bevacizumab is primarily approved for various cancers, it is frequently used off-label for DME because it is a full-length monoclonal antibody that also binds to VEGF and inhibits angiogenesis. It is less expensive than other anti-VEGF drugs, making it a popular off-label choice for treating DME in many countries.
- Faricimab (Vabysmo) Faricimab is a relatively more recent addition approved by the FDA, and it is designed to bind both VEGF-A and Angiopoietin-2 (Ang-2), which makes it unique as a bispecific antibody. By blocking Ang-2, it aids in vascular stability while also providing anti-VEGF activity, potentially allowing for longer treatment intervals between doses.
- Dexamethasone Implant (Ozurdex) This is a corticosteroid formulated as a slow-release intravitreal implant developed by Allergan (now part of AbbVie). It is used to treat DME by reducing inflammation and vascular permeability, and it is particularly helpful for patients who do not respond adequately to anti-VEGF treatments.
- Fluocinolone Acetonide Implant (Iluvien) A long-acting corticosteroid implant that can deliver medication to the eye for up to three years. It offers a sustained treatment option for DME, which may reduce the burden of frequent eye injections for patients.
- Brolucizumab (Beovu) Developed by Novartis, brolucizumab is a relatively new anti-VEGF drug that has been approved for the treatment of wet age-related macular degeneration (AMD) and has been studied in the context of DME as well. Notably, its high concentration and small molecule size were designed to provide a longer duration of action between injections, although there have been concerns regarding potential inflammation-related side effects.
Restoret could potentially fit into the standard of care for DME by providing a new treatment avenue through Wnt pathway modulation. It is positioned to resolve residual fluid and improve visual acuity by restoring and maintaining the blood-retinal barrier, which, if successful in clinical trials, may offer a new and complementary approach to the current anti-VEGF and anti-inflammatory treatments. By acting through a different pathway, Restoret may benefit patients who have an incomplete response to current treatments or might be used in conjunction with these treatments to achieve better patient outcomes.
Neovascular Age-related Macular Degeneration
Neovascular age-related macular degeneration (nAMD), also known as wet AMD, is a chronic eye disorder that results in loss of central vision due to the growth of abnormal blood vessels under the retina that leak fluid and blood. It is a leading cause of severe visual impairment in older adults globally. The market opportunity for nAMD treatments is substantial and growing, driven by an aging population and increasing prevalence of the disease.
Successful drugs for nAMD:The standard of care in nAMD primarily includes anti-VEGF therapies, which have revolutionized the treatment of the disease by significantly reducing the progression of vision loss and, in some cases, even improving visual acuity. Several anti-VEGF agents have been successfully developed and marketed:
- Ranibizumab (Lucentis) – Initially developed for nAMD, this anti-VEGF medication has been successful in the market and widely used across the globe.
- Aflibercept (Eylea) – Another leading anti-VEGF therapy that has displayed long-lasting effects and reduced the frequency of injections needed, improving patient compliance.
- Bevacizumab (Avastin) – This is used off-label for nAMD due to its cost-effectiveness. It has a similar mechanism of action to Lucentis but was originally developed to treat cancer.
- Brolucizumab (Beovu) – A newer anti-VEGF with the ability to provide a longer duration between treatments while offering a similar level of efficacy as other leading treatments.
Standard of Care:Anti-VEGF injections form the centrepiece of nAMD treatment, but the required frequency of these injections can be a burden for patients and healthcare systems.
Specifically:
- Treatment is generally initiated with a monthly injection until maximum visual acuity is achieved or there are no signs of disease activity.
- Following this stabilization period, some patients may be managed with a "treat-and-extend" approach, increasing the interval between injections while monitoring for signs of disease activity.
- In other cases, a "pro re nata" (PRN) strategy is employed, where further treatments are given only when disease activity recurs.
Unmet Medical Need:Despite these therapeutic advances, there are several areas of unmet medical need:
- Frequency of Injections: There is a need for longer-acting therapies that reduce the frequency of eye injections, which would improve patient adherence, decrease caregiver burden, and potentially minimize treatment dropouts.
- Treatment Response Variability: Not all patients respond optimally to anti-VEGF medications, and there's a need for alternatives that can help those who have an inadequate response.
- Durability and Potency: Drugs with greater durability (sustained efficacy over time) and potency may reduce the need for frequent monitoring and treatment.
- Drug Delivery Systems: There is a need for innovative drug delivery systems that provide continuous drug therapy and reduce the requirement for frequent intraocular injections.
- Combination Therapies: Therapies that can be effectively combined with anti-VEGF agents to address multiple pathways involved in the pathogenesis of nAMD could enhance treatment outcomes.
- Advanced Atrophic Changes: For late-stage nAMD, there is an unmet need for treatments that address the geographic atrophy associated with the condition, as well as preventative therapies that can stall disease progression.
Market Opportunity:Given the high prevalence of nAMD in the aging population and the ongoing burden of treatment, there is a substantial market opportunity for innovations that can offer new mechanisms of action, reduce treatment frequency, improve visual outcomes, and offer better long-term disease management. Drugs that address these unmet needs can capture a significant market share.
The pharmaceutical industry, therefore, continues to invest in research for new nAMD treatments that can either complement or provide an alternative to current anti-VEGF therapies. Any new therapies that can answer these unmet needs are likely to be well-received in the market, subject to their safety and efficacy profiles.
There are several promising therapies in development for Neovascular Age-related Macular Degeneration (nAMD), with various approaches aiming to provide either an alternative or an adjunct to the current anti-VEGF treatments. The focus for many of these new treatments is on extending the duration between treatments, reducing the treatment burden, and improving or stabilizing visual acuity. Below are some of the approaches and specific treatments that illustrate the promising developments in the field:
- Port Delivery Systems (PDS) with Ranibizumab This is a surgically implanted device currently being developed by Genentech, which allows for the continuous delivery of a specially formulated version of ranibizumab over an extended period. It aims to reduce the treatment burden associated with frequent intraocular injections.
- Faricimab (Vabysmo) by Roche Faricimab is a bispecific antibody that targets both VEGF and Angiopoietin-2 (Ang-2), aiming to provide broader inhibition of pathways involved in vascular instability. It has been developed for extended dosing intervals in comparison to existing anti-VEGF therapies.
- Brolucizumab (Beovu) by Novartis Brolucizumab is a relatively new anti-VEGF agent that has shown promise in allowing for less frequent dosing intervals. However, safety concerns regarding potential inflammation have been noted, which may impact its use as a first-line therapy.
- Gene Therapies Gene therapies aim to introduce genetic material to produce anti-VEGF proteins directly within the eye, potentially providing a long-lasting treatment option. Examples include RGX-314 being developed by Regenxbio, which uses an adeno-associated virus vector to deliver a gene for anti-VEGF protein production, and ADVM-022 by Adverum, which takes a similar approach.
- Complement Inhibitors These drugs target components of the complement system, which is thought to play a role in the pathogenesis of nAMD. Lampalizumab, an inhibitor of complement factor D, and pegcetacoplan, which inhibits C3, are examples, though trials for lampalizumab have not yielded positive results.
- Integrin Peptide Therapy Integrins are involved in angiogenesis, and therapies that block integrin signaling may inhibit the development of choroidal neovascularization. ALG-1001 (Luminate) is one example of a drug in this class that has undergone clinical trials.
- Tyrosine Kinase Inhibitors (TKIs) These drugs inhibit receptors involved in growth factor signaling pathways related to angiogenesis. Oral tyrosine kinase inhibitors, such as sunitinib, are being investigated for their potential systemic and local effects on nAMD.
- Photodynamic Therapy (PDT) While not a new treatment, the development of improved photosensitizing agents and protocols may make PDT a more effective and feasible option for certain types of nAMD, possibly in combination with anti-VEGF drugs.
- RNA-Based Therapies Antisense oligonucleotides and siRNA therapies designed to knock down the expression of genes involved in angiogenesis and inflammation are under investigation. These could potentially be administered less frequently than current anti-VEGF treatments.
It's important to emphasize that while these treatments show promise, they are all at various stages of development and will require successful completion of clinical trials and regulatory approvals before they can become part of the standard of care for nAMD. The safety, efficacy, durability, and cost-effectiveness of these therapies will ultimately determine their place in the market and their impact on the existing treatment paradigm for nAMD.
If Restoret is effective in clinical trials and gains approval, it may offer several potential advantages that could influence its integration into the standard care for nAMD:
New Mechanism of Action: Restoret may provide an alternative or adjunctive treatment for patients who have an inadequate response to current anti-VEGF therapies.
Potential for Improved Efficacy: By directly targeting the pathway responsible for maintaining the blood-retinal barrier, Restoret could potentially offer a more definitive treatment approach by resolving the residual fluid and improving visual acuity.
Reduced Treatment Burden: If Restoret offers a durable effect, it could potentially decrease the frequency of intravitreal injections and monitoring required for nAMD patients, improving adherence and reducing healthcare resource utilization.
Fulfilling Unmet Needs: The development of Restoret underscores the ongoing demand for new therapeutic options that can provide long-term solutions, reduce treatment frequency, and address cases where current therapies fall short.
The Phase 1b/2a AMARONE clinical trial's completion of multiple ascending dose safety study is a vital step in the development process. The trial aims to demonstrate Restoret's safety and gather preliminary efficacy data. However, it's important to recognize that Restoret's successful integration into the standard of care for nAMD will depend on the outcomes of this and subsequent clinical trials. It needs to show not only efficacy but also a favorable safety profile, considering that intraocular injections carry inherent risks.
If the ongoing and future clinical trials of Restoret yield positive results, EyeBio could potentially establish a new treatment approach to manage nAMD. Such an innovation could reduce the treatment burden associated with frequent anti-VEGF injections, enhance patient outcomes, and address the critical needs in nAMD care. However, the drug's efficacy, safety, tolerability, cost, dosing schedule, and how it compares to existing therapies will all influence its market position and adoption into clinical practice.