Kynexis Therapeutics investment analysis
November 14, 2023
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Overview
Kynexis is a biotechnology company specializing in precision therapeutics for brain diseases, specifically targeting cognitive impairment in schizophrenia. The company raised a €57 million in Series A funding, led by Forbion, in November 2023.
Their principal drug candidate, KYN-5356, is a novel small molecule that inhibits Kynurenine Aminotransferase II (KAT-II), and it is moving towards clinical trials as a potential new treatment for cognitive impairment associated with schizophrenia (CIAS).
The company acquired a worldwide license from Mitsubishi Tanabe Pharma Corporation to develop and market KYN-5356.
Pipeline overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| KYN-5356 | Small molecule | KAT-II inhibitor | Cognitive Impairment Associated With Schizophrenia |
Highlights and risks
Schizophrenia is a large market with significant unmet need.
Targeted, biomarker-driven development could potentially address issues with heterogeneity in patient populations that makes schizophrenia a challenging indication.
Evidence suggests that elevated KYNA levels, which are influenced by KAT-II, are associaetd with cognitive deficits and other symptoms of schizophrenia.
Schizophrenia and psychiatric disorders are very challenging areas of clinical development with low probability of success.
While evidence supports an association between KAT-II, elevated KYNA levels, and cognitive impairment in schizophrenia, KAT-II's direct implication in cognitive impairment is still a subjet of active research.
The safety profile of KAT-II inhibitors, particularly regarding long-term use and potential side effects, is not fully understood. For instance, PF-04859989's development was halted due to cross toxicity concerns.
Valuation
Given the early stage of the company and limited information about its programs, we did not conduct a valuation analysis.
Platform overview
Kynexis's overall scientific strategy focuses on the development of precision therapeutics for brain diseases, specifically targeting cognitive impairment associated with schizophrenia (CIAS). This is a condition that affects millions of individuals and represents a substantial unmet medical need, as current treatments for schizophrenia do not effectively address the cognitive impairments that significantly impact patients’ quality of life.
Their strategy can be outlined as follows:
Human Biology-Based Approach: Kynexis emphasizes the importance of understanding the human biology underlying CIAS rather than relying on animal models, which have traditionally been the mainstay of psychiatric disorder research. This approach is considered more translatable to human diseases and may help in discovering treatments that are more effective.
Causal Biomarker Approach: Kynexis seeks to identify and utilize biomarkers that are causally linked to CIAS to guide their mechanism of action in clinical development. By understanding and targeting these causal biomarkers, they aim to develop treatments with clearer clinical endpoints and objective measures of efficacy.
Human Genetics Approach: The company aims to leverage human genetic data to identify sub-populations that are more likely to respond to their treatments. This stratification can lead to more personalized and effective interventions.
Targeting the Kynurenine Pathway: Kynexis’s lead candidate, KYN-5356, is a potent and highly selective inhibitor of KAT-II, a key enzyme in the kynurenine pathway. Modulation of this pathway is hypothesized to play a significant role in the cognitive aspects of schizophrenia.
The shift from traditional animal models to human biology-focused research is gaining momentum in the field of neuropsychiatric disorders. This trend aligns with the broader movement towards precision medicine, which seeks to tailor healthcare to individual patient characteristics.
Several other companies and research groups are adopting similar human-centered approaches. For example, targeting genetic abnormalities in specific patient populations, often identified through genome-wide association studies (GWAS), has become a common strategy in drug discovery for diseases like cystic fibrosis and certain cancers.
However, there are potential risks and pitfalls with this approach:
Biomarker Validation: Identifying causal biomarkers for diseases, particularly complex psychiatric disorders, is challenging. Biomarkers need to be thoroughly validated to ensure they are truly indicative of the disease and relevant to therapeutic effects.
Complexity of Human Genetics: Human genetic data is complex and variable. While genetics can provide insights into disease mechanisms and potential treatment responses, there is a risk of overestimating the impact of genetic factors to the exclusion of other important aspects such as environment.
Clinical Translation: Even if a treatment is backed by strong human biology rationale and biomarker data, it must still prove itself in clinical trials. The complexity of human diseases means that a treatment that seems promising in early-stage research may not succeed in a heterogeneous patient population.
Ethical Considerations: The use of human genetic data requires careful ethical considerations and adherence to privacy regulations. There is also the risk of creating disparities in treatment availability if only certain sub-populations are identified as likely responders.
In conclusion, Kynexis's approach appears well-founded in contemporary scientific methodologies, increasing the likelihood of successful development of new therapies for CIAS. However, as with all drug development, careful navigation of the scientific and ethical challenges will be crucial for their success.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
KYN-5356
Scientific thesis
The therapeutic rationale for a KAT-II (kynurenine aminotransferase II) inhibitor in the treatment of Cognitive Impairment Associated With Schizophrenia (CIAS) stems from the dysregulation of the kynurenine pathway in schizophrenia, specifically the role this pathway plays in the neuropathophysiology of the disorder, including its influence on cognition.
The kynurenine pathway is a major route of tryptophan metabolism, leading to the production of several neuroactive metabolites, including kynurenic acid (KYNA). Kynurenine aminotransferase II is one of the key enzymes involved in the synthesis of KYNA in the brain. High levels of KYNA have been implicated in cognitive deficits observed in several psychiatric and neurological disorders, including schizophrenia.
Research indicates that elevated concentrations of KYNA may exert neurophysiological effects that could contribute to cognitive deficits. KYNA is an antagonist at the alpha-7 nicotinic acetylcholine receptor (α7nAChR) and the N-methyl-D-aspartate (NMDA) receptor. These receptors are critically involved in synaptic plasticity and cognitive processes. Thus, abnormally high KYNA levels in the brains of individuals with schizophrenia, particularly in critical brain regions for cognition, may impair cognitive function by interfering with the normal function of these receptors.
The lead candidate, KYN-5356, is a potent and highly selective inhibitor of KAT-II, which could modulate the levels of KYNA in the brain, potentially addressing the excessive antagonism at the α7nAChR and NMDA receptors that may contribute to cognitive deficits.
Kynexis is taking a biomarker-based approach, which includes understanding the human genetics associated with CIAS, to identify subpopulations of patients who might respond to this new treatment most effectively. This personalized medicine approach aims to match patients with treatments that address the specific biological underpinnings of their condition, potentially leading to higher efficacy and fewer side effects compared to traditional, one-size-fits-all treatments.
Mechanism of action analysis
The kynurenine pathway's role in schizophrenia is a growing area of interest, but it's not fully established. While there is evidence suggesting this pathway's involvement in schizophrenia, particularly in relation to neuroinflammation and oxidative stress, the exact mechanisms and their contributions to the disorder are still being explored.
The link between high levels of kynurenic acid (KYNA) and cognitive deficits is supported by several studies, but it's important to note that the neuroscience community has not reached a consensus on this. The role of Kynurenine aminotransferase II (KAT-II) in the synthesis of KYNA in the brain is well-established, but its direct implication in cognitive impairment associated with schizophrenia (CIAS) is still a subject of research.
KYNA is known to antagonize α7nAChR and NMDA receptors, which are crucial for synaptic plasticity and cognitive processes. However, the extent to which this antagonism contributes to cognitive deficits in schizophrenia is still debated.
In the last decade, there has been some research into KAT-II inhibitors for schizophrenia. PF-04859989 was shown to be effective in preclinical studies. For instance, it restored glutamate release events in the cortex of rats exhibiting elevated levels of kynurenic acid (KYNA), a metabolite implicated in cognitive symptoms of schizophrenia. The administration of PF-04859989 reversed the effects of elevated KYNA levels, suggesting its potential as a therapeutic compound for cognitive symptoms of schizophrenia and other disorders associated with high brain KYNA level.
Despite the promising preclinical results, PF-04859989 did not proceed to clinical studies. One of the primary reasons was the cross toxicity caused by its irreversible interaction with pyridoxal phosphate (PLP), which is a required cofactor of the KAT isozymes and other PLP-dependent enzymes. This issue of cross-toxicity raised concerns about the safety profile of the compound, which is a critical consideration in the advancement of any therapeutic agent to clinical trials.
The development of KAT-II inhibitors, in general, has faced challenges. While there has been ongoing research on these inhibitors, the number of different chemical types (chemotypes) that have been investigated remains rather low, and none of the inhibitors, including PF-04859989, have advanced into clinical trials. This indicates broader challenges in the field related to the development of safe and effective KAT-II inhibitors for clinical use.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
Market overview
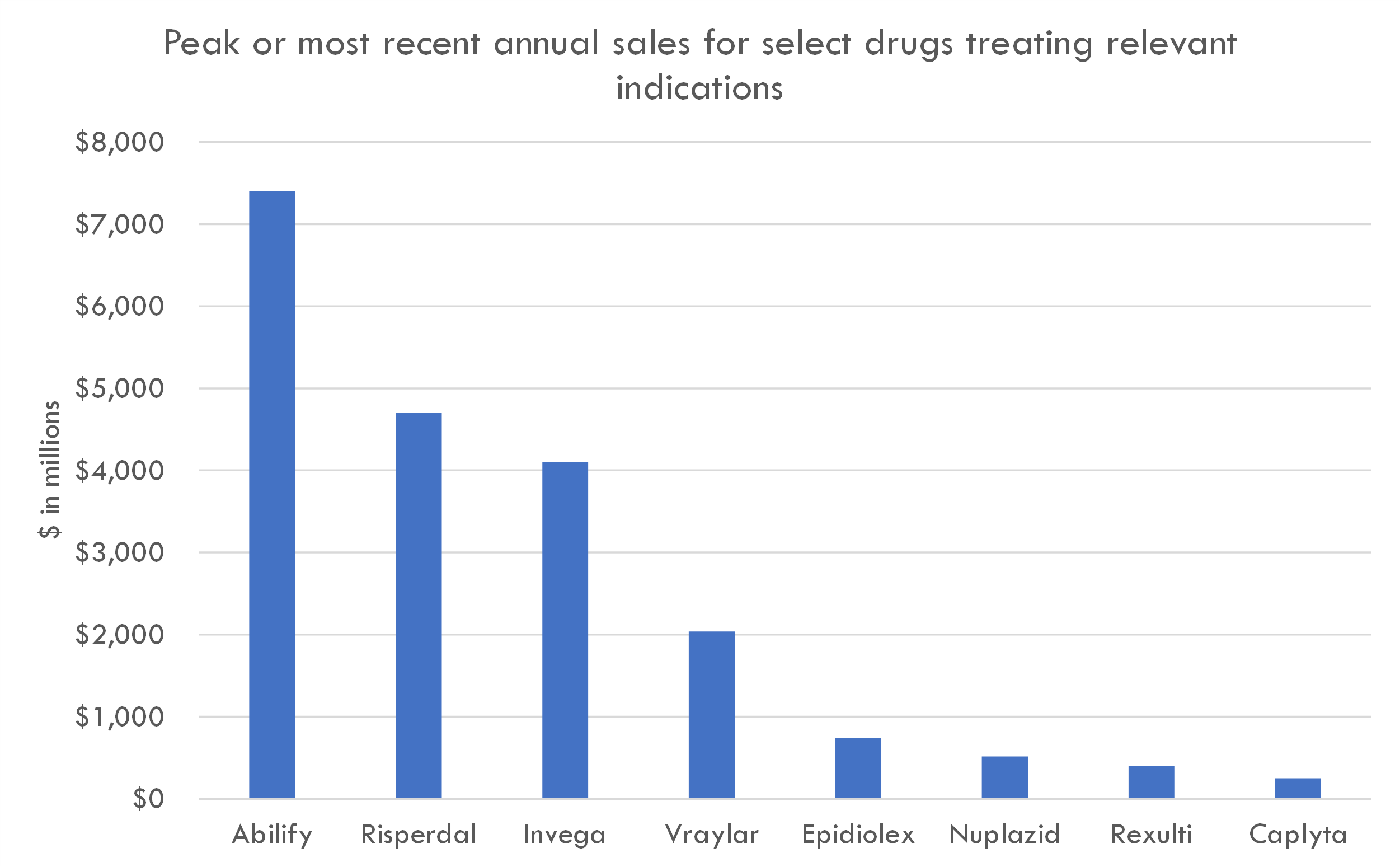
Cognitive impairment associated with schizophrenia (CIAS) is a significant and common symptom in patients with schizophrenia. It can affect various cognitive domains such as memory, attention, executive function, and working memory, leading to functional impairment in patients' daily lives.
Market Opportunity: The market opportunity for treatments targeting CIAS is potentially large for several reasons:
Prevalence of Schizophrenia: Schizophrenia affects approximately 1% of the global population. Given that a substantial proportion of these individuals experience some degree of cognitive impairment, the target population for a CIAS therapy is considerable.
Unmet Medical Need: Currently, there are no drugs approved specifically to treat cognitive impairment in schizophrenia. While antipsychotic medications are the standard of care for schizophrenia, they primarily target positive symptoms such as delusions and hallucinations and have limited efficacy on cognitive deficits. This gap represents a significant unmet medical need and an opportunity for drug developers.
Benefit for Patients: Improving cognitive function in schizophrenia patients aligns with a more comprehensive approach to treating the disease, potentially enabling better functional outcomes and quality of life for patients. This underscores the potential demand for effective treatments.
Other Successful Drugs and Standard of Care: To date, antipsychotics remain the standard of care for schizophrenia, with some commonly prescribed drugs for schizophrenia including olanzapine, risperidone, aripiprazole, and clozapine. These medications can have positive effects on cognitive symptoms, but they are not sufficient for a large number of patients, and cognitive impairment often persists. There are also some non-pharmacological approaches, like cognitive training and cognitive remediation, that are used adjunctively to help manage cognitive deficits.
Unmet Medical Need: The persistent nature of cognitive deficits in schizophrenia and the limited impact of current antipsychotics on these symptoms highlight the significant unmet medical need in treating CIAS. While there are ongoing clinical trials for CIAS with medications intended to directly target cognitive deficits or protect against neurodegeneration, none have yet been approved specifically for the indicational use in CIAS.
Understanding the market potential for such a drug requires accounting for the cost of research and development, regulatory approvals, the competitive environment, potential market penetration rates, pricing strategies, and reimbursement considerations. Given the complexity of the brain and the heterogeneity of schizophrenia as a disorder, developing effective treatments for CIAS is challenging, but breakthroughs in this area could represent a significant advancement in the field and a considerable market opportunity.
Promising Treatment Approaches in Development for CIAS:
Targeting Neurotransmitter Systems: Many clinical studies focus on modulating neurotransmitter systems that are implicated in cognitive functions, such as the cholinergic, glutamatergic, and serotonergic systems. For example, researchers are exploring the potential of alpha-7 nicotinic acetylcholine receptor agonists and muscarinic M1/M4 acetylcholine receptor agonists to improve cognitive symptoms.
Neuroprotective Agents: Oxidative stress and neuroinflammation are thought to play a role in the neuropathology of schizophrenia. Compounds with neuroprotective properties that can mitigate these pathological processes may have the potential to prevent or improve cognitive impairment in schizophrenia.
NMDA Receptor Modulators: The NMDA (N-Methyl-D-Aspartate) receptor, a subtype of glutamate receptor, is believed to be involved in the pathophysiology of CIAS. Compounds that act as modulators, such as positive allosteric modulators, may improve cognitive impairments by enhancing NMDA receptor function.
BDNF Enhancers: Brain-Derived Neurotrophic Factor (BDNF) is important for synaptic plasticity, which is essential for learning and memory. Compounds that can enhance BDNF signaling might have the potential to ameliorate cognitive deficits in schizophrenia.
GABA Modulators: Alterations in GABAergic (gamma-aminobutyric acid) neurotransmission have been associated with cognitive symptoms in schizophrenia. Modulating the GABA system is another approach being explored in preclinical studies.
Histone Deacetylase Inhibitors: Epigenetic modifications have been implicated in schizophrenia, and histone deacetylase (HDAC) inhibitors have shown potential in preclinical studies for their neuroprotective properties and ability to affect gene expression related to cognitive function.
mTOR Pathway Modulators: The mTOR (mechanistic target of rapamycin) signaling pathway is involved in synaptic plasticity and memory formation. Preclinical research indicates that targeting this pathway could be beneficial in treating cognitive impairment.
Metabolic Modifiers: As energy metabolism abnormalities might contribute to cognitive dysfunction, agents that can influence mitochondrial function or overall cellular metabolism may offer a novel approach to treatment.
Immunomodulators: Preclinical evidence suggests a role for immune dysregulation in the pathophysiology of schizophrenia, including cognitive impairment. Immunomodulatory drugs are being investigated for their potential to target neuroinflammation and improve cognitive outcomes.
Many antipsychotic drugs can also have some impact on cognitive symptoms, but this is typically a secondary benefit rather than the primary indication. It's also important to note that the effect of antipsychotics on cognitive symptoms can be quite variable among individuals.
However, many pharmaceutical and biotechnology companies are actively researching and developing drugs to target CIAS. These are some potential types of drugs that may be in development, though none are approved specifically for CIAS:
Cognitive Enhancers or Nootropics: These are drugs or supplements that are purported to improve cognitive function, specifically executive functions, memory, creativity, or motivation, in healthy individuals. An example is modafinil, which is used for narcolepsy but has been researched for cognitive effects in schizophrenia.
Alpha-7 Nicotinic Acetylcholine Receptor Agonists: Drugs that target this specific receptor subtype are being investigated for their potential to enhance cognitive functioning, given the role of cholinergic systems in cognition.
Glutamatergic System Modulators: This includes drugs that modulate NMDA receptors or the broader glutamatergic system, which could help to address the hypothesized glutamate dysfunction in schizophrenia.
Additionally, drug repurposing—where existing drugs approved for other conditions are tested for new therapeutic uses—is a common approach to finding treatments for CIAS, due to the known safety profiles of established drugs.