Kyverna Therapeutics IPO investment analysis
January 16, 2024
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or an ongoing business relationship with the company.
Kyverna Therapeutics is a clinical-stage biopharmaceutical company developing innovative cell therapies for autoimmune diseases. Utilizing CD19 CAR T-cell technology, the company's proprietary approach draws insight from successful applications in other medical areas to deliver therapeutic benefits for autoimmune conditions.
The leading candidate, KYV-101, is an autologous CD19 CAR T-cell therapy licensed from the NIH, which has demonstrated higher tolerability compared to existing oncology CAR-T therapies in Phase 1 trials. Structural optimizations in the CAR construct's hinge and membrane domains contribute to its potential in treating autoimmune diseases by depleting disease-contributing B cells.
Kyverna is targeting diseases such as lupus nephritis (LN), systemic sclerosis (SSc), myasthenia gravis (MG), and multiple sclerosis (MS), with IND clearances for Phase 1/2 and Phase 2 studies indicating a robust development pipeline. Preliminary academic clinical data suggests that CD19 CAR T-cell therapies can induce remission in autoimmune diseases, providing a strong foundation for Kyverna's trials.
Given the high prevalence of autoimmune diseases and the limitations of current treatments, Kyverna has the potential to meet a significant unmet medical need. With the global autoimmune therapy market exceeding $80 billion, there is considerable potential for impactful innovation.
Kyverna's portfolio includes KYV-201, an allogeneic CD19 CAR T-cell product in partnership with Intellia Therapeutics, expanding into other autoimmune conditions such as inflammatory bowel diseases (IBD). Collaborations with research institutions and novel manufacturing initiatives, like Ingeni-T, emphasize Kyverna's multifaceted strategy to enhance patient experiences and production capabilities, positioning the company to pioneer advancements in cell therapy treatments for autoimmune diseases.
In summary, Kyverna Therapeutics is leveraging CAR T-cell therapies—validated in oncology—to potentially reset the immune system for sustained remission in various autoimmune conditions. The firm's ongoing research into regulatory T cells (Tregs) and novel CAR constructs highlights its innovative edge in the growing autoimmune therapy market.
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| KYV-101 | Cell therapy | CD19 CAR-T | Lupus nephritis | |||||||
| KYV-101 | Cell therapy | CD19 CAR-T | Systemic sclerosis | |||||||
| KYV-101 | Cell therapy | CD19 CAR-T | Myasthenia gravis | |||||||
| KYV-101 | Cell therapy | CD19 CAR-T | Multiple sclerosis | |||||||
| KYV-201 | Cell therapy | CD19 CAR-T | Undisclosed autoimmune diseases |
Strong rationale for CD19 CAR-T in autoimmune diseases where B-cells are implicated
CD19 CAR-T anti-B-cell activity validated through oncology applications
Encouraging, though early, initial clinical data
Bar for safety of CAR-T in autoimmune disease is higher than in oncology; early safety data encouraging but larger, longer-term studies could unveil issues
CAR-T therapies are expensive, logistically complex therapies and would need compelling efficacy data to justify high price
Unclear how durable response will be, and whether re-administration would be feasible given cost, complexity and potential toxicity of therapy
The role of B cells varies across autoimmune conditions, and it is unclear whether therapy would be effective across range of autoimmune diseases
Valuation
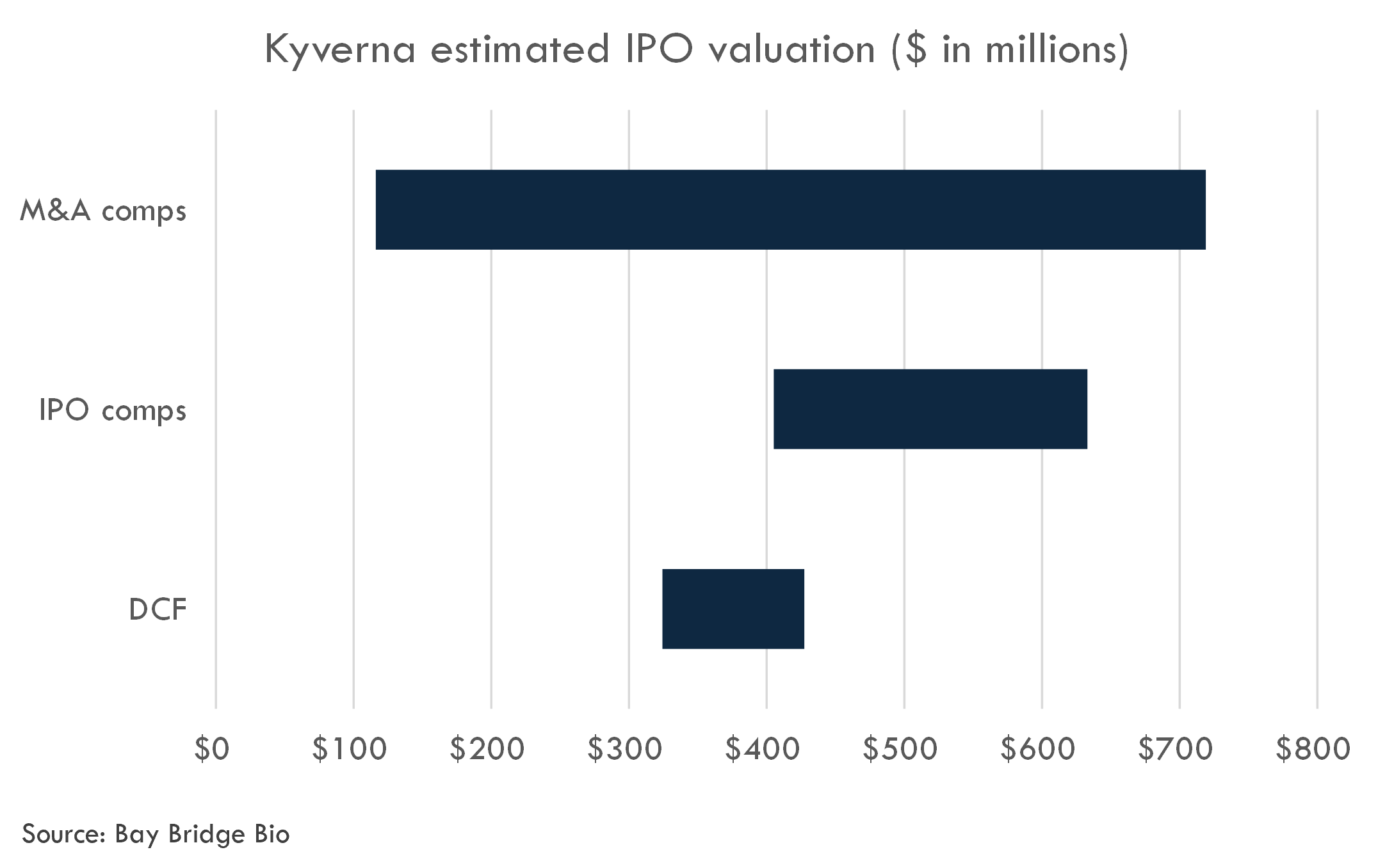
We estimate Kyverna's last private round valuation was $242 million. We estimate the fully diluted IPO post-money valuation at $405-633 million.
KYV-101
Therapeutic rationale
Autologous CD19 CAR-T cell therapies, such as KYV-101, represent a novel approach to treating autoimmune diseases by targeting and modifying patients' own immune cells to combat the disease. In autoimmune diseases like lupus nephritis, systemic sclerosis (SSc), myasthenia gravis (MG), and multiple sclerosis (MS), the body's immune system mistakenly attacks its own tissues, causing various degrees of inflammation and damage. The therapeutic rationale for using CD19 CAR-T cell therapy in these conditions is based on the pivotal role of B cells in the pathogenesis of autoimmune diseases.
B cells are central players in the immune system and can act as both effectors and regulators of immune responses. In many autoimmune diseases, B cells contribute to pathology through the production of autoantibodies, presentation of autoantigens to T cells, and secretion of pro-inflammatory cytokines. Targeting CD19, a surface molecule expressed on B cells, enables the selective elimination of these cells, thereby reducing the autoimmune activity.
For lupus nephritis, which is a severe manifestation of systemic lupus erythematosus (SLE) affecting the kidneys, the CD19 CAR-T cells could be advantageous by removing autoantibody-producing B cells, leading to a reduction in immune complex deposition and inflammation in the kidneys. Clinical case studies have shown that CD19 CAR-T therapy can lead to remission in SLE patients refractory to other treatments, along with a decrease in autoantibody levels and proteinuria.
In systemic sclerosis (SSc), B cells contribute to fibrosis by producing profibrotic cytokines and autoantibodies that can lead to vascular and tissue damage. CD19 CAR-T cells may interrupt these B cell-mediated processes and potentially halt or reverse disease progression.
For myasthenia gravis (MG), an autoimmune disease characterized by autoantibodies against components of the neuromuscular junction, CD19 CAR-T cell therapy may eliminate these autoantibody-producing cells, potentially improving neuromuscular function.
In multiple sclerosis (MS), B cells are thought to contribute to disease pathology both within the central nervous system and peripherally. Depleting B cells via CD19 CAR-T therapy may reduce the autoimmune response against myelin and neuronal elements, potentially reducing disease activity and progression.
KYV-101 has been optimized to enhance the safety profile of CAR-T therapy. By utilizing a fully human scFv, KYV-101 aims to minimize the development of anti-CAR immune responses, which could be more prevalent in autoimmune diseases due to hyperactive immune systems. This design may offer improved persistence and expandability, crucial for long-term efficacy, and allow for the possibility of retreatment if necessary.
Further, the modified construct utilized in KYV-101 is designed to reduce cytokine release and potentially lessen the incidence and severity of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which are significant adverse effects associated with CAR-T therapies.
Overall, the rationale for utilizing autologous CD19 CAR-T cell therapy in these autoimmune diseases is to selectively target and eliminate pathological B cells with an improved safety profile, thereby reducing autoimmune activity and related damage without compromising the broader immune system to a significant extent. Clinical results in oncology and autoimmune case studies indicate the promise of KYV-101 and similar therapies, but more data from clinical trials in autoimmune disease populations will be necessary to fully establish efficacy and safety profiles for these novel treatments.
The science behind using CD19 CAR-T cell therapy for the treatment of autoimmune diseases is currently experimental and represents a new frontier extending beyond the initially established use of CAR-T therapies in oncology, particularly B-cell malignancies, where these treatments have been successfully applied and are FDA-approved.
Key Points with Established Science:
- CD19 as a B-cell marker: The expression of CD19 on B cells is well-established, and the role of B cells in autoimmune diseases is recognized, particularly as producers of autoantibodies and as antigen-presenting cells that can perpetuate autoimmune responses.
- CAR-T efficacy in oncology: The success of CD19-targeted CAR-T therapies in treating certain hematologic cancers, such as B-cell lymphomas and leukemias, has confirmed the principle of CAR-T technologies in selectively targeting and eliminating cells expressing specific antigens.
Uncertainties and Scientific Debate:
- Long-term Efficacy: While initial case reports and small studies suggest that CD19 CAR-T therapy can lead to remission in some autoimmune diseases, the durability of these responses and long-term efficacy remain uncertain. Larger, controlled trials with extended follow-up periods are required to establish the sustainability of these therapeutic effects.
- Safety Profile: The side effects known in oncological use of CAR-T therapies, such as CRS and ICANS, have been reported to be less severe with KYV-101. However, the side effect profile and long-term safety of these therapies in the context of autoimmune diseases—where patients may have systemic inflammation and organ involvement—are still under investigation.
- Applicability Across Autoimmune Diseases: While there is a theoretical rationale for using CAR-T therapy across various autoimmune diseases, the exact mechanisms of each condition differ, and the role B cells play can vary greatly. It remains unclear which diseases will respond best to CD19 CAR-T therapy.
- Immune System Reset: The idea of "resetting" the immune system through B-cell depletion followed by its reconstitution is an area of great interest but also of debate. Whether newly generated B cells post-treatment represent a truly "reset" immune component without autoimmune potential is a question being investigated.
- Impact on Long-term Immunity: Concerns about how B-cell depletion and modification might affect long-term immunological memory, vaccine responses, or increase the risk of infections are still being explored.
The overall level of evidence for CD19 CAR-T therapy's effectiveness and safety in autoimmune diseases is low compared to its use in oncology, primarily relying on case reports, small case series, and initial clinical trials. There is a need for ongoing research to validate and expand upon the preliminary findings that have been reported. The evidential support for processes such as immune system reset is still emerging, and while the potential is recognized, definitive conclusions await results from ongoing and future studies.
CD19 is a pan-B cell marker expressed from the earliest B cell precursors to the B cell blasts, though not on plasma cells. The role of CD19 in autoimmune diseases arises from its presence on B cells, which contribute to these conditions through various mechanisms such as autoantibody production, antigen presentation, and cytokine secretion. Below are summarized points addressing the role of CD19 and its involvement in several autoimmune diseases, supported by scientific literature:
Lupus Nephritis (LN):
The role of B cells in systemic lupus erythematosus (SLE) and by extension, lupus nephritis, is well-established. B cells are implicated in the pathogenesis of the disease due to the production of autoantibodies and immune complex formation, which can deposit in the kidneys, causing lupus nephritis. Rituximab, an anti-CD20 monoclonal antibody that depletes B cells, has been explored in SLE and LN, indicating the significance of B cells in these conditions.
- There is literature demonstrating rituximab's potential in LN, such as the LUNAR trial, though it did not meet its primary endpoint (Rovin BH et al., Kidney International, 2012).
- Case studies have documented that CD19-targeted CAR-T cells can induce remission in refractory SLE cases (Mackall CL, Fry TJ, Gress RE, et al., Nature Reviews Disease Primers, 2020).
Systemic Sclerosis (SSc):
B cells are implicated in systemic sclerosis pathogenesis due to their roles in fibrosis and autoimmunity. Abnormal B cell activation and autoantibody production are hallmarks of the disease.
- Autoreactive B cells and specific autoantibodies are frequently observed in patients with SSc (Sato S et al., Autoimmunity Reviews, 2005).
- B cell depletion therapies have been used with some success, supporting the involvement of B cells. Case reports have recorded improvements in skin fibrosis and pulmonary function following B cell depletion therapy (Daoussis D, Liossis SN, Tsamandas AC et al., Arthritis and Rheumatism, 2010).
Myasthenia Gravis (MG):
In MG, autoantibodies attack components of the neuromuscular junction, leading to muscle weakness. B cells are the source of these pathogenic autoantibodies.
- Treatments aimed at reducing autoantibody production, such as rituximab, have shown effectiveness in MG (Hehir MK, Hobson-Webb LD, Benatar M, et al., Neurology, 2020).
- A case report of autologous CD19-targeted CAR-T cells in refractory MG demonstrated clinical improvement, suggesting a potential role for this therapeutic strategy (Braunstein MJ et al., JCI Insight, 2020).
Multiple Sclerosis (MS):
B cells contribute to the pathogenesis of MS through autoantibody production and the secretion of proinflammatory factors and are present in the CNS of people with MS.
- The efficacy of B cell depletion therapies such as ocrelizumab, an anti-CD20 monoclonal antibody, supports the role of B cells in MS (Hauser SL, Bar-Or A, Comi G, et al., New England Journal of Medicine, 2017).
- Studies and reports involving the use of CAR-T cells for treating MS are more limited compared to other autoimmune diseases, and the treatment's role in MS is still exploratory.
It's worth noting that literature directly linking CD19 CAR-T to therapeutic outcomes in these autoimmune diseases is sparse and primarily limited to case reports and small case series. Much of the rationale for using CD19 CAR-T therapy in autoimmune conditions is derived from the known contribution of B cells to these diseases and the efficacy of other B cell depletion strategies, which serves as an indirect foundation for CD19-targeted therapy. As the field is burgeoning, future research and broader clinical trials will be important to substantiate the role of CD19 CAR-T cell therapy in these conditions and generate more robust literature support.
The therapeutic rationale for using autologous CD19 CAR-T cell therapy in autoimmune diseases is formulated on the basis of several key pillars:
Strengths of the Evidence Base:
- Role of B cells in Autoimmunity: There is a well-established body of evidence implicating B cells in the pathogenesis of autoimmune diseases. B cells can contribute to these conditions through autoantibody production, antigen presentation, and cytokine release, all of which are pivotal in sustaining the autoimmune responses.
- Success in B-Cell Malignancies: The clinical efficacy of CD19 CAR-T cell therapies in B-cell malignancies such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) is well-documented. This success provides a strong proof of concept for the ability of CAR-T cells to effectively target and eliminate B cells.
- Targeting CD19: CD19 is a sensible target for B-cell-directed therapies due to its consistent expression on B cells throughout their development, except for plasma cells. This makes CD19 a reliable antigen for directing therapy against the entire B-cell lineage.
- Case Reports and Small Studies: Case reports and small studies have shown promise for CD19 CAR-T therapy in autoimmune diseases. Examples include reports on the successful treatment of refractory SLE with durable remission, reduction in autoantibody levels, and significant clinical improvement.
- B-Cell Depletion Therapies: The effectiveness of B-cell depletion therapies like rituximab in some of the autoimmune diseases provides indirect support. Although rituximab targets CD20 and not CD19, its mechanism of action similarly involves the depletion of B cells, thus giving credibility to strategies targeting B-cell surface molecules.
Weaknesses of the Evidence Base:
- Limited Data in Autoimmune Disorders: The majority of evidence comes from the oncology setting, with limited clinical trial data available for autoimmune diseases. The serious nature of adverse events associated with CAR-T therapies in oncology necessitates caution and thorough investigation in less life-threatening conditions like autoimmune diseases.
- Lack of Large-Scale Studies: Most evidence supporting CD19 CAR-T therapy in autoimmune diseases comes from case reports and small, uncontrolled studies. Large-scale, randomized controlled trials are needed to validate these findings and assess the balance of risks and benefits.
- Durability of Response: The long-term clinical benefits and durability of B cell depletion via CAR-T cells in the context of autoimmunity are still unknown. Concerns remain regarding the re-emergence of autoreactive B cells after treatment.
- Potential Overgeneralization: Different autoimmune diseases have varying pathophysiological mechanisms, and what is effective in one disease may not be translatable to another. Thus, the rationale for using CD19 CAR-T cells based on evidence from one autoimmune disease may not be universally applicable.
- Safety Profile: CAR-T cell therapy is associated with significant adverse events such as CRS and ICANS in oncology. The safety profile relating to autoimmune diseases is still not well characterized, bringing uncertainty to its application in these patients who may have different tolerance levels for such side effects.
- Impact on Normal Immunity: Concerns about the possible impact of long-term B cell depletion on normal immune function, vaccination response, and increased infection risk remain open questions due to limited follow-up data.
In summary, while the rationale for using CD19 CAR-T therapy in autoimmune diseases is grounded in scientific theory and preliminary findings, the evidence base for its clinical application in these conditions is still developing. Large-scale, controlled clinical trials, longer-term safety and efficacy data, and a better understanding of the immunological effects of prolonged B cell depletion are necessary to strengthen the evidence supporting this therapeutic approach.
Clinical trials
Phase 1 in lupus nephritis
Study Design Summary:
The clinical trial is a Phase 1, interventional, open-label, multicenter study investigating KYV-101, in subjects with refractory lupus nephritis (LN). KYV-101 is an autologous fully-human anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy. The estimated enrollment is 12 adult participants.
The subjects receive KYV-101 CAR-T cell therapy following a standard lymphodepletion regimen (cyclophosphamide and fludarabine). The primary purpose of the study is treatment, and the interventional model consists of sequential assignment, meaning different groups of participants may receive the treatment in a specific sequence. Outcomes are assessed up to 24 months post-treatment.
Primary Outcome Measures:
- Incidence of adverse events (AEs) and laboratory abnormalities.
- Frequency of dose-limiting toxicities at each dose level.
Secondary Outcome Measures:
- Pharmacokinetics of KYV-101 CAR-positive T cells in blood.
- Pharmacodynamics, including B cell and cytokine levels in blood.
- Disease-related biomarkers (anti-dsDNA, complement C3, C4 levels in serum).
- Efficacy measures such as complete renal response rates and time to disease worsening.
- Immunogenicity analysis by determining the development of anti-KYV-101 antibodies by immunoassays.
Critiques of the Study Design:
- Sample Size: With only 12 participants, it might be challenging to draw conclusive evidence about the safety and efficacy of the therapy.
- Lack of Control Group: The open-label design without a control group (such as a placebo or standard-of-care arm) means there is no comparison for evaluating the true effect size or placebo response.
- Single-Arm Trial: Due to the sequential assignment in a single-arm trial, there might be temporal biases if the external conditions change during the course of the study.
- Open-Label Bias: Open-label studies can introduce bias because participants and researchers are aware of the treatment being administered, which may influence reporting of outcomes and adherence to the treatment protocol.
Operational and Technical Challenges:
- CAR-T Cell Manufacturing: The production of CAR-T cells is complex and requires sophisticated facilities. Ensuring consistent quality and managing logistics can be challenging, especially with multiple study sites.
- Patient Monitoring: The potential for serious adverse events requires intensive post-infusion monitoring and access to medical care capable of managing these events.
- Lymphodepletion: The conditioning regimen is cyto-reductive and may induce significant side effects, requiring careful management.
- Disease Assessment: Accurately determining clinical response in lupus nephritis can be complex due to the heterogeneous nature of the disease.
- Regulatory and Ethical Considerations: As this is a new therapeutic approach, there may be additional regulatory scrutiny and the need for comprehensive informed consent due to the potentially irreversible nature of gene therapy.
- Immunogenicity: As KYV-101 is a fully human CAR-T cell therapy, the risk of immunogenicity may be lower, but it is still crucial to monitor for any anti-drug antibody response.
Overall, the study is innovative in its approach to treating refractory lupus nephritis. However, the small size and open-label design may limit the generalizability of the findings, and the technical complexity of CAR-T cell therapy, including ensuring patient safety, presents significant operational challenges.
Proof-of-Concept Potential:
This study aims to evaluate KYV-101 in the specific context of refractory lupus nephritis. The stringent selection criteria, including a requirement for biopsy-proven proliferative lupus nephritis and a confirmed diagnosis of systemic lupus erythematosus (SLE), provide a targeted cohort that is appropriate for proof of concept. The primary endpoints, which include the incidence of adverse events and the frequency of dose-limiting toxicities, are well-chosen for a Phase 1 trial, where safety is the principal concern. Secondary endpoints relating to pharmacokinetics, pharmacodynamics, and efficacy indicators like complete renal response rates and time to disease worsening are appropriate for assessing the initial hints of KYV-101’s therapeutic effect.
Appropriateness of Primary and Secondary Endpoints:
The primary endpoint focusing on AEs and safety is a standard for Phase 1 trials and is critical when dealing with a new therapy such as CAR-T, which can have significant side effects. Secondary endpoints aim to assess the biological activity of KYV-101, such as the depletion of B cells, levels of inflammatory cytokines, and specific disease markers like anti-dsDNA, which contribute to the underlying lupus nephritis pathology. Evaluating complete renal response rates and changes in disease biomarkers provides a direct measure of clinical efficacy and correlates with patient outcomes.
Inclusion Criteria:
The criteria set to select patients are detailed and require confirmed cases of lupus nephritis, thus ensuring that the participants indeed have the disease that the therapy aims to treat. These stringent criteria will likely enhance the quality of the data regarding the drug’s efficacy and safety. Vaccination currency is a sensible requirement, given that immunocompromised patients are at higher risk for infections, and lymphodepleting regimens could exacerbate this risk.
Exclusion Criteria:
The exclusion criteria are extensive to restrict the study to a population that will not have confounding factors that could influence the interpretation of KYV-101’s effects or substantially increase the risk to patients. For example, excluding patients with active infections (like hepatitis B/C and HIV) or a history of certain neurological conditions prevents enrolling individuals who could have compromised outcomes not directly related to the drug’s mechanism of action. Similar considerations are made with the exclusion of patients with a history of malignancies, cell or gene therapy, and stem cell transplants.
Reproducibility Challenges:
Strict inclusion and exclusion criteria may enhance internal validity but could also limit the external validity and the generalizability of the findings. By excluding a broad range of potentially confounding or risk-enhancing factors, the trial may not represent the broader population of lupus nephritis patients who have comorbidities or previous treatments that are part of real-world settings. This selectiveness might challenge the reproducibility of the results in more diverse clinical populations.
One of the potential challenges for reproducibility also lies in the need for consistency and precision in CAR-T cell manufacturing. The autologous nature of the therapy means each batch of CAR-T cells is patient-specific, and any variations in manufacturing could potentially influence reproducibility and outcome measures.
In summary, the eligibility criteria are likely to ensure a homogenous study population ideal for a proof-of-concept study, but the same factors that strengthen internal validity might pose challenges to the scalability and applicability of the findings across the broader lupus nephritis population.
Interim results in lupus nephritis
The clinical data supporting KYV-101 in Lupus Nephritis (LN) includes two clinical trials, KYSA-1 (U.S.-based) and KYSA-3 (Germany-based). These trials aim to assess the safety, pharmacokinetics, and efficacy of KYV-101 in patients with refractory LN.
Key Points from the KYV-101 Clinical Development:
- Study Design:
- KYSA-1: 12 adult patients, open-label, multi-center.
- KYSA-3: Phase 1 with 6-12 patients, Phase 2 with up to 20 patients.
- Primary Endpoints:
- Incidence of adverse events and laboratory abnormalities.
- Frequency of dose-limiting toxicities.
- Complete Renal Response (CRR) rate (Phase 2 of KYSA-3).
- Secondary Endpoints:
- Pharmacokinetics and pharmacodynamics assessment.
- Evaluation of disease-related biomarkers.
- Efficacy measurements such as time to CRR and CRR.
- Evaluation of immunogenicity.
- Patient Enrollment and Dosage:
- First patient dosed in KYSA-1 in July 2023.
- First patient dosed in KYSA-3 in November 2023.
- Objective Clinical Endpoints:
- Proteinuria measured by Urinary Protein-Creatinine Ratio (UPCR) as an indicator of LN disease activity.
- CRR is a quantitative, objective composite endpoint for trials and includes resolution of proteinuria.
- Early Clinical Results (as of December 31, 2023):
- Improvement in UPCR in the first two patients in KYSA-1 and the first patient in KYSA-3.
- Patient from KYSA-1 experienced a UPCR decrease from 1.5 to under 0.5 by day 120 without immunosuppressives or steroids.
- Patient from KYSA-1 (SLE for two years) showed a UPCR improvement from 3.4 to 0.6 by day 30.
- In KYSA-3, a patient's UPCR improved from 2.5 to 1.1 by day 27.
- Adverse Events:
- Grade 1 Cytokine Release Syndrome (CRS) was observed and manageable with acetaminophen in two KYSA-1 patients.
- No Immune Cell-Associated Neurotoxicity Syndrome (ICANS) or other serious adverse events were reported.
- Prolonged CD19+ B-cell depletion was noted, with neutrophil, hemoglobin, and platelet levels normalizing within weeks.
- Additional Biomarkers:
- Measures of anti-dsDNA antibodies and complement levels (C3 and C4) help assess disease activity in lupus.
- Safety Profile:
- Consistent with NIH Phase 1 observations in oncology patients treated with the same CAR construct, serious grade CRS and ICANS have not been observed in the LN patients treated.
In conclusion, the clinical data shows that KYV-101 has been well-tolerated in early Lupus Nephritis cases with improvements in disease-related biomarkers, providing a promising therapeutic potential with a positive safety profile. However, it is important to note that these results are preliminary and based on a small patient cohort. Further data from these trials will be necessary to fully evaluate the safety and efficacy of KYV-101 in treating Lupus Nephritis.
In the context of Lupus Nephritis (LN), the design of clinical trials and the selected endpoints are critical for the evaluation of a new therapy's efficacy and safety, and ultimately for its approval by regulatory bodies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Based on the clinical and scientific literature, as well as previous trials for similar drugs, here are some considerations for the approvable endpoints, clinical studies, and patient numbers required for a therapy like KYV-101.
Approvable Endpoints:- Primary Endpoints:
- Complete Renal Response (CRR): Defined as a UPCR of ≤0.5, normal or near-normal (within 10% of normal if previously abnormal) kidney function as measured by eGFR (estimated glomerular filtration rate), and the absence of active urinary sediment.
- Major Clinical Response (MCR): Defined similarly to CRR but allows for a higher level of proteinuria (e.g., UPCR <1.0) in some cases.
- Partial Renal Response (PRR): Defined as a specified percentage reduction in proteinuria (e.g., ≥50%) and stability or improvement in renal function.
- Secondary Endpoints:
- Reduction in anti-dsDNA antibody levels: Often correlated with disease activity.
- Normalizing complement levels (C3 and C4): Low levels are often associated with active disease.
- Health-related quality of life scores (HRQoL): Using validated questionnaires.
- Reduction or cessation of steroid use: Reliance on steroids is a surrogate marker for disease activity.
- Durability of response: Sustained CRR, MCR, or PRR over a given time period.
- Exploratory Endpoints:
- Biomarkers for LN: Such as new urinary or blood biomarkers that correlate with disease activity.
- Biopsy data: Histopathological improvements in kidney tissue, if biopsies are ethically and safely attainable.
- Phase 2 Trials:
- These trials are often exploratory and aim to establish proof-of-concept efficacy and dose-ranging information. Patient numbers can vary widely but typically range from several dozen to a few hundred.
- Phase 3 Trials:
- These are larger, pivotal studies that provide definitive evidence of efficacy and safety. They are designed to confirm Phase 2 findings and are required for drug approval.
- Usually randomized, double-blinded, and placebo-controlled to provide the highest level of evidence.
- The size of Phase 3 trials varies but often involves several hundred to a few thousand patients to ensure adequate power to detect differences between treatment groups.
- Long-term Extension Studies:
- To assess the long-term safety and efficacy. The size can be similar to or larger than Phase 2 trials and may last several years.
- Given the variability in the progression of LN and responses to treatment, these trials need to be adequately powered to detect clinically meaningful effects. For a Phase 2 trial, a few dozen to a few hundred patients might be needed. For Phase 3 trials, several hundred to a few thousand patients might be required.
- It’s important to note that these numbers can vary greatly depending on the specific trial design, the statistical power needed, the expected effect size, patient population, and variability of the response.
- The design of these trials will also depend on regulatory feedback. For example, the FDA often provides specific guidance for the development of drugs for complex diseases like LN, and might require certain endpoints or study designs based on the most current scientific understanding of the disease.
- Finally, regulators might also prefer composite endpoints that capture multiple aspects of the disease since LN has a variety of clinical presentations and can affect patients differently. The use of a composite endpoint can help ensure that the treatment has a broad beneficial effect on the disease.
Systemic sclerosis
The study being described is designed to assess the safety, tolerability, and clinical activity of a fully-human anti-CD19 CAR T-cell therapy (KYV-101) in adult subjects with B cell-driven autoimmune diseases, including idiopathic necrotizing myopathy (INM), diffuse cutaneous systemic sclerosis (dcSSc), systemic lupus erythematosus (SLE) with nephritis, and ANCA-associated vasculitis (AAV).
The trial is interventional and Non-Randomized with parallel assignment and will enroll 24 participants, with six participants in each disease group. They will all receive a single dose of 1.0×10[8] CAR+ T cells after receiving lymphodepleting chemotherapy consisting of cyclophosphamide and fludarabine. Participants will be followed for two years.
Critiques of the study design could include:
- Small Sample Size: Each group consists of only six participants, which may limit the statistical power of the study and the ability to generalize findings to a larger population.
- Diversity of Conditions: Since different autoimmune diseases may respond differently to the treatment, having a multi-disease study may complicate the interpretation of results.
- Non-Randomized Design: Without randomization, there may be biases in participant selection which could affect the study outcomes.
- Open Label: Lack of blinding can introduce observer bias, which may affect the reporting of results, particularly subjective assessments.
Operational or technical challenges specific to this study design:
- Manufacturing and Delivery: Producing CAR T-cells is technically complex and must be done in a highly controlled environment, ensuring each batch is consistently produced to specific guidelines.
- Patient Monitoring: Close monitoring is required due to potential severe adverse events associated with CAR T-cell therapy, requiring specialized medical infrastructure and staff.
- Infusion Reactions: Administering KYV-101 may lead to infusion-related reactions which will require immediate response measures.
The primary outcome measures are the incidence and severity of adverse events (AEs) in each participant group at various time points post-CAR T-cell infusion (3 months, 6 months, 12 months, and 24 months). This concentration on safety is typical for a phase 1 clinical trial, where the main goal is to evaluate the safe dose range and identify side effects.
Overall, the study is designed to provide initial data on whether KYV-101 may be a safe and potentially effective new treatment option for patients with these B cell-driven autoimmune diseases, with a significant focus on monitoring for any adverse effects.
The potential of this study to provide proof-of-concept for the use of KYV-101 in Systemic Sclerosis (SSc) and other autoimmune diseases is primarily demonstrated through the assessment of safety and tolerability, which are suitable primary endpoints for a Phase 1 trial. The inclusion of clinical activity measures as secondary endpoints would help to determine the preliminary efficacy of the treatment.
For diseases like SSc, endpoints often involve quantification of skin involvement, such as the Modified Rodnan Skin Score (MRSS), or assessments of internal organ involvement, such as pulmonary function tests for interstitial lung disease (ILD). It's important to note that while the primary endpoints are focused on the incidence and severity of adverse events (AEs), secondary endpoints assessing disease-specific clinical activity would be essential for evaluating the potential efficacy of KYV-101 in Systemic Sclerosis and other conditions included in the trial.
Inclusion and exclusion criteria are crucial for ensuring a consistent and specific patient population, which helps in attributing the effects of the treatment to the actual therapeutic intervention rather than underlying differences in the study participants. The criteria listed are detailed and tie the eligibility of participants to well-defined clinical and laboratory measures, which could increase the homogeneity of the participant groups and the reliability of the study outcomes. However, this thoroughness presents several potential reproductive challenges:
- Stringent criteria may limit enrollment: The comprehensive inclusion/exclusion criteria may restrict the number of participants eligible for the study, making it more challenging to enroll enough patients, especially for rarer conditions.
- Generalizability: While strict criteria improve internal validity by ensuring a homogenous study population, they can limit the external validity, or generalizability, of the study results to all patients with the diseases under study.
- Operational complexity: The need for recent biopsies, specific tests, and various assessments prior to enrollment can complicate the study's logistics and may introduce potential delays in initiating treatment.
- Consistency: Ensuring the adherence to such detailed criteria across different centers, if the study is expanded beyond a single center, could be challenging and might impact the study's reproducibility and consistency of data.
As for the study's specific focus on Systemic Sclerosis, the primary endpoints might not fully inform on the progression or improvement of the disease, since SSc is multi-faceted and affects multiple organ systems. The study includes both skin and lung involvement in the inclusion criteria, which are common manifestations of SSc, but other organ systems are not explicitly accounted for in the primary outcome measures.
Inclusion in the study requires a diagnosis based on validated criteria, moderate to severe disease activity according to specific measures, and refractory disease after multiple treatments. These criteria will help select subjects for whom conventional therapies are not working and for whom KYV-101 may offer a benefit. This population represents a significant unmet medical need, highlighting the potential impact of the study findings.
In summary, the study design has the potential to provide an initial proof-of-concept for KYV-101 in Systemic Sclerosis; however, its specific efficacy endpoints and inclusion criteria may affect its ability to be reproduced widely, and any findings may need further validation in larger, more diverse patient populations.
Myasthenia gravis
Study Summary:
KYV-101 is an investigative therapy for Myasthenia Gravis (MG), particularly for patients who have refractory generalized myasthenia gravis. This study is designed to evaluate the effectiveness and safety of KYV-101, a fully human anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. It will explore the potential of these CAR-T cells to deplete both normal and autoreactive B-cells, which are implicated in the pathogenesis of MG due to their role in producing autoantibodies that affect the neuromuscular junction, leading to muscle weakness.
The study is an open-label, multicenter, phase 2 trial with a single group assignment. All participants will undergo a standard lymphodepletion regimen, followed by dosing with KYV-101 CAR-T cells. The estimated enrollment for the study is 20 subjects, with the study start date anticipated in February 2024 and with primary completion expected by May 2026.
Critiques of Study Design:
- The trial is open-label (non-blinded), which may introduce bias in reporting outcomes as both researchers and participants are aware of the treatment being administered.
- The single group assignment without a control group makes it difficult to compare the treatment's effectiveness against standard of care or placebo, which could affect the interpretation of the efficacy results.
- As the study is not yet recruiting, the sample size is relatively small (20 participants), which may limit the statistical power and generalizability of the study conclusions to a broader population.
Operational and Technical Challenges:
- Production and Quality Control: Manufacturing of CAR-T cells is complex and requires stringent quality control measures. Ensuring that each batch of KYV-101 cells meets the criteria for safety and efficacy is critical but challenging.
- Logistics of CAR-T Therapy: The process of leukapheresis, cell modification, and reinfusion requires careful coordination between treatment centers and manufacturing facilities, potentially leading to logistical hurdles.
- Lymphodepletion: The necessity of a lymphodepletion regimen prior to infusion of CAR-T cells can be associated with significant side effects, requiring careful monitoring and management.
- Monitoring for Adverse Effects: CAR-T therapies can trigger severe immune reactions, such as cytokine release syndrome (CRS). Close monitoring and rapid intervention for adverse effects will be vital.
- Assessing Long-Term Outcomes: The study design includes long-term follow-up for up to 24 months. Long-term tracking of patients' health status can be operationally challenging and may be complicated by loss to follow-up.
- Immunogenicity: There is a potential for patients to develop antibodies against the CAR-T cells, which could impact the treatment's efficacy and safety.
- Measuring Outcomes: The study will assess the efficacy using specific MG scores (i.e., MG-ADL, QMG, MGC) and changes in disease-related antibody levels. However, these measures may not fully capture the patient's overall health or quality of life adjustments, necessitating the inclusion of broader assessment tools.
Potential for Proof-of-Concept:
This study is designed to explore the proof-of-concept for the use of KYV-101 in myasthenia gravis (MG), with a specific goal to show that targeted depletion of B cells by CAR-T cells can be an effective treatment for the condition. The inclusion criteria allow for the selection of patients who have a confirmed diagnosis of MG and are in a particular spectrum of disease severity (MGFA Class IIB-IV). The presence of specific autoantibodies (AChR and MuSK) in participants ensures that the CAR-T cells are targeting relevant immunological components in MG.
Appropriateness of Primary and Secondary Endpoints:
The primary endpoints focus on the safety and tolerability of KYV-101 as assessed by the incidence of adverse events and laboratory abnormalities, which are appropriate for early-phase clinical trials. Additionally, the ability of KYV-101 to improve daily living activities as measured by the Myasthenia Gravis Activities of Daily Living (MG-ADL) total score is a direct and meaningful clinical endpoint for efficacy.
Secondary endpoints include measures of muscle strength (QMG score), overall disease severity (MGC score), and autoantibody levels (AChR, MuSK, and LRP4 antibodies). These provide a more comprehensive assessment of the therapy's impact on MG and will allow the researchers to correlate clinical outcomes with immunological changes. The pharmacokinetic and pharmacodynamic measurements, such as CAR-positive T cell counts and B cell counts, will provide insights into how the therapy engages with the immune system and its durability over time.
Inclusion/Exclusion Criteria:
The inclusion criteria effectively target patients who may potentially benefit the most from CAR-T therapy by focusing on those with antibody-positive, moderate to severe MG. Excluding patients with a broad range of neurological disorders and severe comorbidities helps to isolate the effects of KYV-101 and reduces the risk of complications that could confound the results.
Potential Reproducibility Challenges:
- Patient heterogeneity: MG can be quite variable in presentation and response to treatment. This heterogeneity could affect the reproducibility of the study's results across different patient populations.
- Rigorous exclusion criteria: While necessary for ensuring patient safety and reducing variability, the stringent exclusion criteria could make it difficult to recruit participants, and the results may not be representative of all MG patients, particularly those with comorbidities or more complex disease presentations.
- Monitoring and assessment consistency: Given the reliance on clinical scores to assess outcomes, standardized training and strict adherence to assessment protocols are necessary to ensure reproducibility across different centers.
- Manufacturing consistency: Producing CAR-T cells with consistent quality and characteristics is essential. Variations in the final product could influence the therapy’s efficacy and safety.
Conclusion:
The study has the potential to provide evidence for the efficacy and safety of KYV-101 in treating MG, using appropriate clinical endpoints. The selection criteria are well-suited to limit confounding factors and patient risk, though they may pose some challenges in terms of patient recruitment and generalizability. Further, reproducibility of the results could be challenging due to the variability in disease presentation and the complexity of CAR-T cell production.
Clinical data to date
The clinical data supporting KYV-101 for the treatment of Myasthenia Gravis (MG) is derived from named patient case reports, which document individual patient experiences rather than results from formal clinical trials. Still, these data provide valuable insights into the potential therapeutic effects of KYV-101 in MG patients:
- Patient Outcomes:
- The first patient treated with KYV-101 showed no KYV-101-related adverse events.
- A significant (70%) reduction in pathogenic autoantibodies was observed by day 62 post-infusion, while protective vaccination IgG levels were maintained.
- An improvement in muscle strength was noted, with enhanced walking ability, a lower Besinger disease activity score, and reduced quantitative MG (QMG) scores.
- Second Patient Results:
- The second patient also tolerated KYV-101 well, with only low-grade CAR T-cell adverse events, including moderate flu-like symptoms representing Grade 1-2 CRS, handled with standard treatments, and Grade 1 ICANS.
- Post-treatment, successful B-cell depletion, reduced autoantibody levels, and muscle strength recovery were reported.
- Notably, this patient progressed from wheelchair dependency to bicycling within two months and began mountain touring after four months.
- Overall Progress:
- A total of six MG patients had been treated with KYV-101 on a named patient basis as of December 31, 2023.
- The reduction in QMG scores for the first two patients was positive, suggesting an improvement in MG disease activity.
- Publication and Presentation of Data:
- The case of the first patient has been published in Lancet Neurology.
- Results from the second patient were presented at both the 96th Congress of the German Society of Neurology and the American Academy of Neurology conference.
Despite the promising outcomes indicated in these reports, these case studies cannot be directly used in applications for marketing approval with regulatory agencies such as the FDA. However, they provide a valuable source of preliminary evidence that could inform the design and de-risk future controlled clinical trials by Kyverna. Controlled studies will still be necessary to confirm the efficacy, safety, and optimal dosing of KYV-101 in a broader MG patient population. The evidence from the named patient treatments supports continuing research and suggests that KYV-101 may offer a significant benefit for patients with severe and refractory MG.
For a therapeutic agent like KYV-101 targeting Myasthenia Gravis (MG), there are established endpoints for clinical trials informed by scientific literature, regulatory guidance, and previous clinical trial designs for similar or competing drugs. The endpoints are essential for demonstrating the efficacy and safety of the drug for regulatory approval.
Approvable Endpoints for KYV-101 in MG:
- Primary Efficacy Endpoints:
- Quantitative Myasthenia Gravis (QMG) score: This scale is widely used in MG trials to measure the disease severity based on quantifiable muscle strength assessments.
- Myasthenia Gravis Activities of Daily Living (MG-ADL) score: A patient-reported outcome tool that quantifies the impact of MG on daily activities.
- Secondary Efficacy Endpoints:
- Myasthenia Gravis Composite (MGC) score: A shorter, composite score of MG symptoms and functions.
- Myasthenia Gravis Quality of Life (MG-QoL) questionnaire: A patient-reported outcome measure assessing quality of life.
- Reduction or discontinuation of immunosuppressive drugs: This could include corticosteroids, which are a mainstay of MG management and whose reduction can signify stable disease improvement.
- Muscle-specific kinase (MuSK) autoantibody levels: For patients with MuSK MG, the reduction in autoantibody titers may correlate with clinical improvement.
- Safety Endpoints:
- Incidence of adverse events: Including treatment-related adverse events, serious adverse events, and adverse events leading to discontinuation of treatment.
- Changes in laboratory parameters: Can include immunoglobulin levels, complete blood count, and liver enzymes.
- Occurrence of CAR T-cell therapy-related complications: Such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
Clinical Studies They May Need to Conduct for Approval:
- Phase 1/2 Trials: Designed to assess the safety profile, pharmacokinetics, pharmacodynamics, and preliminary efficacy of KYV-101. These trials might involve a dose-escalation study to determine the optimal dose with the best safety and efficacy balance.
- Phase 3 Trials: Randomized, double-blind, placebo-controlled trials to confirm the efficacy and safety findings from Phase 2. These are typically larger, multi-center studies that provide definitive evidence for regulatory approval.
- Long-term Follow-up Studies: To monitor long-term safety and durability of the treatment effect.
Estimated Number of Patients Required for These Studies:
- Phase 1/2 Trials: These early-phase trials might include anywhere from 20 to 100 patients, depending on the trial's design, stratified by various disease subtypes and severity.
- Phase 3 Trials: For rare diseases like MG, Phase 3 trials might be smaller than those for common diseases and could involve several hundred patients. The actual size will depend on the statistical power needed to demonstrate a clinically and statistically significant difference between treatment arms.
- Long-term Follow-up Studies: For cell therapies like CAR T-cells, long-term safety is crucial, and these studies may continue for several years post-treatment, potentially including all patients who received the therapy.
The precise number of patients will depend on anticipated effect sizes, variability in response, and the specific endpoints chosen. The study design will also be informed by discussions with regulatory agencies and key opinion leaders in the field, and may be influenced by the evolving landscape of MG treatment and regulation.
Multiple sclerosis
The clinical data for KYV-101 in Multiple Sclerosis (MS) is part of a broader developmental program and named patient treatments covering various autoimmune conditions. Here's a summary of the key points:
- Patient Dosing and Follow-up:
- As of December 31, 2023, 14 patients had been dosed with KYV-101, and 13 of these patients had reached a 28-day follow-up. This group includes patients being treated for multiple sclerosis as well as other autoimmune diseases.
- Safety Observations:
- The key safety observations revolve around cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Reports suggest that patients with autoimmune diseases tolerated CAR T-cell therapy well, experiencing lower-grade CRS and ICANS when compared to oncology patients.
- There were no serious CRS or ICANS toxicities observed in the autoimmune patients after being dosed with KYV-101. Specifically mentioned are six MG patients, three lupus nephritis patients, two MS patients, one patient with stiff person syndrome (SPS), and one patient with anti-DAGLA encephalitis (DE).
- Comparisons with Oncology and Other CD19 CAR T-cell Therapies:
- Patients treated with other CD19 CAR T-cell therapies exhibited Grade 3 or higher CRS and ICANS primarily in an oncology setting. The CD19 CAR used in other therapies reportedly contains murine binders, whereas KYV-101 uses a fully human binder.
- Despite the observations of better tolerability in autoimmune conditions, the information comes from case reports and separate clinical settings, and there is no definitive head-to-head comparison data available for autoimmune versus oncology indications.
- CAR T-cell Expansion Data:
- Early clinical experience with KYV-101 suggests no clinically meaningful differences in the kinetics or extent of CAR T-cell expansion compared to previous CAR T cells employing the identical Hu19-CD828Z CAR, based on data from an NIH Phase 1 trial.
- The Hu19-CD828Z CAR is consistent across both the autoimmune patients treated with KYV-101 and DLBCL patients treated in NIH trials with the same CAR.
- Cautions Regarding Future Clinical Data:
- Although current observations are promising, future clinical results, including those from sponsored KYSA-1 and KYSA-3 trials and other studies, might not confirm the early safety observations documented. Caution is advised when drawing conclusions from these preliminary data.
In summary, KYV-101 seems to have a favorable safety profile based on early observations in a variety of autoimmune diseases, including multiple sclerosis. Low-grade CAR-related safety events were manageable, and no serious CRS or ICANS events were reported in the treated autoimmune patient cohort. However, these results are from a limited number of patients and will require confirmation in larger, controlled, and potentially randomized clinical trials to validate these findings and secure regulatory approval.
For a therapeutic agent such as KYV-101 to be approved for Multiple Sclerosis (MS), it must demonstrate efficacy and safety through clinical studies designed around endpoints that reflect meaningful change in disease activity and patient quality of life. The clinical endpoints and study designs are informed by scientific literature, regulatory guidance, and clinical precedent from similar or competing drugs.
Possible Approvable Endpoints for KYV-101 in Multiple Sclerosis:
- Primary Efficacy Endpoints:
- Relapse Rate: Reduction in the annualized relapse rate compared to baseline or placebo.
- Disability Progression: Measured by changes in the Expanded Disability Status Scale (EDSS); a prolonged time to sustained progression of disability is often used.
- MRI Activity: The number of new or enlarging T2 lesions and gadolinium-enhancing lesions on MRI as a measure of inflammation and disease activity.
- Secondary Efficacy Endpoints:
- Brain Volume Change: Assessment of brain atrophy over time as a surrogate marker for neurodegeneration.
- Quality of Life Measures: Using validated scales such as the Multiple Sclerosis Quality of Life-54 (MSQOL-54) or the Multiple Sclerosis Impact Scale (MSIS-29).
- Cognitive Function: Assessed with tests such as the Symbol Digit Modalities Test (SDMT) or the Paced Auditory Serial Addition Test (PASAT).
- Safety Endpoints:
- Incidence of Adverse Events: Including treatment-related adverse events, serious adverse events, and events leading to discontinuation.
- Laboratory Parameters: Monitoring of blood counts, liver enzymes, inflammatory markers, and immune cell profiles.
- CAR T-cell Therapy Associated Toxicities: Specifically monitoring for CRS and ICANS given their association with CAR T-cell therapies.
Clinical Studies for Approval:
- Phase 1 Trials: These are typically early-stage trials focused on assessing safety, tolerability, and dosing. For MS, this would likely be a small trial with fewer than 50 patients.
- Phase 2 Trials: Mid-stage trials designed to get preliminary efficacy data along with further safety information. These might include several dozen to a few hundred patients, depending on the effect size and variability expected.
- Phase 3 Trials: These are the large, pivotal trials intended to provide robust evidence for both efficacy and safety. These trials might require several hundred to a few thousand patients and are usually randomized, placebo-controlled, and double-blinded to minimize bias.
- Long-term Extension or Post-Marketing Studies: To accumulate data on the long-term safety and sustained efficacy of the treatment, potentially including all treated patients over multiple years.
Estimated Number of Patients Required for These Studies:
The needed size of each trial phase can vary widely in MS research:
- Phase 1 Trials: Often small, with approximately 20 to 50 patients to determine safety and initial dosing.
- Phase 2 Trials: Can range from 50 to several hundred patients, depending on the trial's objectives.
- Phase 3 Trials: Could include several hundred to several thousand patients, depending on expected disease variability, predicted effect size, and the precision needed to demonstrate statistically significant differences in the primary endpoints.
- Long-term Studies: This population might encompass all or a subset of participants from earlier phases, tracked for extended periods.
The design and size of these clinical trials will also be guided by previous trials of other CAR T-cell therapies, the natural history of MS, and regulatory requirements. The estimated number of patients will also factor in the rate of disease progression, the heterogeneity of the MS patient population, and any stratification needed to account for different types of MS, such as relapsing-remitting MS (RRMS) versus progressive forms. Each stage of clinical development will require close interaction with regulatory bodies to ensure the study design meets the necessary criteria for eventual drug approval.
Market overview
Lupus nephritis
Lupus nephritis is an inflammation of the kidneys caused by systemic lupus erythematosus (SLE), which is an autoimmune disease. In SLE, the immune system mistakenly attacks healthy tissue leading to inflammation and damage to various parts of the body, including the kidneys.
The pathology of lupus nephritis involves the deposition of immune complexes (antibodies bound to antigens) in the glomeruli, which are the filtering units of the kidneys. This immune complex deposition triggers an inflammatory response that can lead to kidney damage. The inflammation can cause various degrees of glomerular injury, which is classified into six different classes (I-VI) based on the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification system. This system considers factors like the degree of immune deposits and cellular proliferation.
Lupus nephritis can manifest through various symptoms, many of which may not appear until the condition has progressed. Common symptoms include:
- Blood in the urine (hematuria)
- Foamy urine due to excess protein (proteinuria)
- High blood pressure (hypertension)
- Swelling in legs, ankles, or feet (edema)
- Elevated levels of creatinine or urea in the bloodstream
- Fatigue and general malaise
These symptoms may be accompanied by other signs of SLE, such as joint pain, skin rashes, fever, and serositis (inflammation of membranes lining the chest and abdomen).
Diagnosis typically involves a combination of blood and urine tests to assess kidney function and may include a biopsy of kidney tissue to determine the extent and type of damage present.
The prognosis for lupus nephritis varies depending on the severity and response to treatment. Early diagnosis and aggressive treatment improve outcomes. If left untreated or if treatment is ineffective, lupus nephritis can lead to end-stage renal disease (ESRD), requiring dialysis or kidney transplantation.
Treatments target reducing inflammation and suppressing the immune system to prevent further damage. Common medications include corticosteroids, immunosuppressive agents (such as cyclophosphamide, mycophenolate mofetil, or azathioprine), and more recently biologics like Rituximab. Additionally, hydroxychloroquine is often used for its protective effects against SLE flares. Treatment may also involve managing symptoms and preventing complications, such as hypertension and cardiovascular disease, with appropriate medications.
In summary, lupus nephritis is a serious complication of SLE that can lead to significant kidney damage and even renal failure if not adequately treated. Effective management combines immunosuppression, symptomatic treatment, and monitoring for potential side effects of therapy. The prognosis has improved with advances in treatment, but lupus nephritis still remains a major cause of morbidity among individuals with SLE.
KYV-101 represents an investigational therapy that targets B cells in the treatment of various autoimmune diseases, including lupus nephritis. The market opportunity for KYV-101 in lupus nephritis can be assessed by examining the existing treatment landscape, the unmet medical needs in the indication, and potential advantages KYV-101 may offer over current therapies.
Standard of Care and Existing Treatments:
The standard of care in lupus nephritis includes a range of pharmacological treatments aimed at reducing inflammation and controlling the immune response. These include corticosteroids, immunosuppressive drugs such as cyclophosphamide, mycophenolate mofetil, azathioprine, and the newer biologic agents like belimumab and rituximab. More recently, voclosporin, an immunomodulating agent, has been approved for the treatment of lupus nephritis in combination with a background immunosuppressive therapy regimen.
Unmet Medical Need:
Despite the availability of these treatments, there remains a significant unmet medical need. Not all patients respond to existing therapies, and there is a subset of patients who are refractory to current treatments (estimated at 40,000 in the US). Moreover, the existing therapies are not curative and many have substantial side effects, which can lead to poor patient compliance and quality of life. Additionally, treatments such as biologics and calcineurin inhibitors can carry risks for serious infections, malignancies, or renal toxicity. Given these challenges, there is a demand for more effective and safer therapies that can provide long-term disease control with fewer adverse events.
Market Opportunity for KYV-101:
KYV-101 offers the potential to meet this unmet medical need by providing a targeted B-cell depletion therapy, which could translate into improved efficacy and safety profiles. The use of chimeric antigen receptor (CAR) T-cell therapy, specifically directed at CD19-expressing B cells, represents an innovative approach to addressing the pathophysiology of lupus nephritis.
The market opportunity is significant, given the chronic nature of lupus nephritis, the number of patients with the disease, and the need for better treatment options. If KYV-101 demonstrates superior efficacy and safety in clinical trials, it could capture a sizable share of the lupus nephritis market. Based on the higher prevalence of SLE and its frequent progression to lupus nephritis, this could represent a considerable patient population eligible for treatment.
Comparative Analysis:
The approval of Ocrevus (ocrelizumab), an anti-CD20 monoclonal antibody, for multiple sclerosis supports the role of B cells in autoimmune diseases and underscores the potential for therapies such as KYV-101 that target B cells. CD19 CAR T-cell therapy might offer deeper B-cell depletion and the possibility of a more durable treatment response compared to monoclonal antibodies, as it could potentially reset the immune system. This could be especially relevant for refractory lupus nephritis patients who have not responded to other lines of therapy.
Conclusion:
Considering the substantial autoimmune disease therapy market, the observed increase in autoimmune conditions, and the high number of patients not optimally responding to current therapies, KYV-101 could fulfill a critical gap in lupus nephritis management. Its success will depend on clinical trial outcomes, demonstrating the benefits of B-cell depletion and the ability to reset the immune system with an acceptable safety profile. If KYV-101 can achieve this, it would address the significant unmet needs in lupus nephritis and possibly command a premium in the market, given its potential advantages over existing therapies.
Several promising treatments for lupus nephritis are in development and could potentially compete with KYV-101. Given the complexity and variability of the disease, various approaches are being explored, targeting different aspects of the pathological process. Here are some categories of competitors that KYV-101 may face upon potential approval:
Monoclonal Antibodies:
- Rituximab (Rituxan): An anti-CD20 monoclonal antibody that depletes B cells; it has shown mixed results in lupus nephritis but remains a potential treatment option, particularly for refractory cases.
- Belimumab (Benlysta): The first biologic approved for SLE that targets B-lymphocyte stimulator (BLyS); it's used in combination with standard therapies and has shown benefits in reducing disease activity.
- Obinutuzumab (Gazyva): Another anti-CD20 monoclonal antibody, which has been shown to be more potent than rituximab in depleting B cells, is under investigation for lupus nephritis and may offer enhanced efficacy over existing therapies.
Small Molecule Therapies:
- Voclosporin: This novel calcineurin inhibitor has been designed for stability and potency with a reduced side-effect profile. It has been approved in combination with a background immunosuppressive therapy regimen for the treatment of lupus nephritis and shows significant promise.
- Baricitinib: A Janus kinase (JAK) inhibitor that interferes with the signaling pathway of various cytokines and has demonstrated positive results in a phase 2 trial for lupus nephritis.
Cell Therapies:
- Mesenchymal Stem Cell (MSC) Therapy: MSCs have regenerative properties and can modulate the immune system, reducing inflammation, which might be beneficial in treating lupus nephritis.
- Other CAR T-cell Therapies: Additional companies are exploring different CAR T-cell constructs that target B cells, utilizing either CD19 or other antigens relevant to the autoimmune disease pathology.
Stem Cell Transplantation:
Although still somewhat experimental for autoimmune diseases, hematopoietic stem cell transplantation (HSCT) is being evaluated in severe cases of lupus nephritis. This treatment involves ablating the patient's immune system and reconstituting it with stem cells free from the memory of the autoimmune response.
The competitive landscape underscores the importance of KYV-101 demonstrating strong efficacy, safety, and tolerability in clinical trials. It must offer clear advantages over these existing and emerging therapies, whether through improved clinical outcomes, fewer side effects, or better patient quality of life. Additionally, factors like cost, convenience of administration, and reimbursement will play critical roles in determining its success in the market.
To succeed against this backdrop, the development and marketing strategy for KYV-101 will need to carefully consider how it differentiates from these alternatives, potentially by showcasing its ability to provide a more durable response or by demonstrating a favorable side-effect profile compared to standard therapies. Additionally, market access strategies, patient support programs, and strong collaboration with key stakeholders will be essential to ensure KYV-101's uptake and success, should it receive regulatory approval.
There are several notable drugs used to treat lupus nephritis, including both long-established treatments and more recently approved medications. Here is an overview of some key drugs:
- Corticosteroids: Prednisone is a common corticosteroid used to reduce inflammation and suppress the immune system in lupus nephritis patients.
- Immunosuppressants: Drugs like cyclophosphamide and mycophenolate mofetil (CellCept) are used to control the immune system's attack on the kidneys. Azathioprine (Imuran) is another immunosuppressant that may be used as maintenance therapy following induction treatment with stronger agents.
- Hydroxychloroquine: Although not used specifically to treat lupus nephritis, hydroxychloroquine is commonly prescribed to help manage overall systemic lupus erythematosus (SLE) activity, which can help control nephritis.
- Calcineurin Inhibitors: Tacrolimus and ciclosporin are calcineurin inhibitors that have been used off-label in the management of lupus nephritis.
Recent approvals for lupus nephritis treatment, which signify significant progress in the field, include the following:
- Belimumab (Benlysta): Developed by GlaxoSmithKline (GSK), belimumab was the first biologic treatment approved for SLE and later received an additional approval specifically for lupus nephritis. It is a monoclonal antibody that inhibits the B-lymphocyte stimulator (BLyS) protein and thereby reduces B cell activity, which is implicated in the development of lupus. The approval for lupus nephritis treatment was granted by the U.S. Food and Drug Administration (FDA) in December 2020.
- Voclosporin (Lupkynis): This is a novel calcineurin inhibitor developed by Aurinia Pharmaceuticals and was approved by the FDA in January 2021 for the treatment of adult patients with active lupus nephritis. Voclosporin is used in combination with a background immunosuppressive therapy regimen, and clinical trials have demonstrated its efficacy in achieving renal response and reducing proteinuria.
- Rituximab (Rituxan): Although rituximab has not been officially approved by the FDA for lupus nephritis, it is used off-label for this condition. Rituximab depletes CD20-positive B cells and has shown effectiveness in some patients with lupus nephritis, particularly those who do not respond to other treatments.
These drugs represent a mix of treatment mechanisms addressing the overactive immune response characteristic of lupus nephritis. The increased understanding of the disease has led to the development of targeted therapies that show promise in improving patient outcomes. As the field continues to evolve, it is expected that new treatments will become available, offering hope for better management of this challenging disease.
KYV-101, as an autologous CAR T-cell therapy targeting CD19-expressing B cells, represents a novel approach in the treatment of lupus nephritis (LN). Its clinical development suggests it may offer a significant advancement in the management of LN, especially for patients with refractory disease who do not respond adequately to current standard of care treatments.
In the context of lupus nephritis, the standard of care typically includes corticosteroids, various immunosuppressants, and more recently, biologics such as belimumab and the calcineurin inhibitor voclosporin. These treatments help manage the disease to varying degrees, but challenges with efficacy, safety, and patient response remain. KYV-101 could potentially fit into this spectrum as a next line of therapy for patients who do not achieve remission with these treatments.
In the early clinical development reports for KYV-101, positive changes in urine protein-creatinine ratio (UPCR) have been observed, suggesting an improvement in kidney function and LN activity. Similarly, the observed absence of serious cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) indicates a manageable safety profile in the early phases of its development. These aspects are critical as safety concerns are paramount when it comes to the application of CAR T-cell therapies in chronic conditions like autoimmune diseases.
KYV-101 may potentially offer several advantages that could make it an attractive addition to the standard of care for LN, including:
- Durable Response: CAR T-cell therapies theoretically offer a long-lasting response with a single treatment by reprogramming the patient's immune cells to continuously fight the disease.
- Targeted Therapy: By selectively targeting B cells, the therapy may reduce off-target effects and toxicity related to broader immunosuppression.
- Biomarker-driven Indication: The use of objective biomarkers like UPCR and complement levels to measure efficacy aligns with the trend towards personalized medicine, making it easier to identify responders and monitor disease activity.
- Reduced Need for Immunosuppressive Drugs: If KYV-101 can maintain remission without the need for ongoing immunosuppression, it may offer a better safety profile and quality of life for patients.
- Revolutionizing Refractory Patients' Treatment: There is a significant need for effective treatments among patients who are refractory to current therapies, which KYV-101 is initially targeting. This population has limited options, and a successful CAR T-cell therapy could be transformational.
It's important to note that CAR T-cell therapy is currently an intense and resource-heavy treatment, typically associated with high costs and significant infrastructure requirements. Its application in LN will need to address such challenges to be broadly adoptable.
Overall, if KYV-101 continues to demonstrate efficacy and safety in larger clinical trials and ultimately gains FDA approval, it may offer a compelling treatment for patients with refractory LN, fitting in as either an alternative or an adjunct to current standard of care therapies. The actual place in therapy would depend on the outcomes of ongoing trials, how well it addresses unmet needs in terms of efficacy and safety, and how it is perceived by healthcare providers and payers in terms of cost-effectiveness and overall value proposition in LN management.
Systemic sclerosis
Systemic sclerosis, also known as scleroderma, is a chronic, complex, and often progressive autoimmune disease primarily characterized by hardening and tightening of the skin ("sclerosis") due to overproduction of collagen and other extracellular matrix components. However, systemic sclerosis also affects internal organs and can lead to widespread vascular and fibrotic changes.
The precise cause of systemic sclerosis remains unclear, but the pathology involves three major intertwined processes: vascular damage, immune system activation, and fibrosis.
- Vascular damage is one of the earliest manifestations, leading to endothelial dysfunction and vasculopathy. It results in abnormalities in the small blood vessels and capillaries, leading to Raynaud's phenomenon and potentially severe blood flow issues to various parts of the body.
- Immune system activation: Systemic sclerosis appears to be mediated by an abnormal immune response, with autoimmunity playing a central role. There is production of autoantibodies against various cellular components, endorsing systemic inflammation and tissue damage.
- Fibrosis: Activation of fibroblasts, which are cells that produce collagen and other fibers, leads to the excessive deposition of extracellular matrix components in the skin and internal organs. This causes thickening and hardening of tissues and impairs their function.
Symptoms can vary in severity and range from mild to life-threatening. Common symptoms include:
- Skin changes: Hardening and tightening of the skin, particularly on the hands, arms, and face, which can restrict movement.
- Raynaud's phenomenon: Extremities such as fingers and toes turn white and blue in the cold due to restricted blood flow, often accompanied by pain and tingling upon warming or stress.
- Gastrointestinal issues: Difficulty swallowing, acid reflux, and motility problems due to fibrosis in the gastrointestinal tract.
- Musculoskeletal symptoms: Joint pain, stiffness, and swelling.
- Pulmonary involvement: Development of interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH), which are leading causes of death in systemic sclerosis patients.
- Cardiac issues: Fibrosis can affect the heart muscle, leading to arrhythmias, heart failure, and pericarditis.
- Kidney involvement: Renal crisis, characterized by sudden-onset hypertension and renal failure.
The prognosis for patients with systemic sclerosis varies greatly and is influenced by the extent and severity of the organ involvement, particularly the lungs, heart, and kidneys. Lifespan may be reduced, especially in those who develop major organ complications. However, with better understanding, early diagnosis, and improved therapeutic strategies, outcomes have been improving.
Diagnosis involves a combination of clinical evaluation of symptoms, physical examination of the skin, blood tests for specific autoantibodies, and assessments of organ function, including pulmonary function tests and echocardiograms.
Treatment focuses on managing symptoms and slowing disease progression but is not curative. Options include vasodilators for Raynaud's phenomenon, proton pump inhibitors for acid reflux, and immunosuppressive agents like mycophenolate mofetil and cyclophosphamide to reduce inflammation and fibrosis. For particularly severe cases, treatments such as hematopoietic stem cell transplantation or targeted therapies like the anti-fibrotic agent nintedanib (for ILD) may be considered.
Systemic sclerosis is a highly heterogeneous condition with distinct clinical manifestations and disease courses, making individualized patient care essential. Recent advancements in understanding the molecular underpinnings and the treatment landscape offer new hope for patients suffering from this often debilitating disease.
KYV-101, a CAR T-cell therapy targeting CD19, presents a potential market opportunity in the treatment of systemic sclerosis (SSc) based on its immunomodulatory mechanism of action, particularly if it can ameliorate the disease's autoimmune component by addressing B-cell activity. Standard care and current pharmaceutical developments are crucial to appreciate this market potential fully.
Standard of Care:
The current treatment landscape for SSc is limited. Standard of care options primarily focus on managing symptoms and preventing complications through the use of immunosuppressants (like cyclophosphamide and mycophenolate mofetil for pulmonary involvement), vasodilators (for Raynaud's phenomenon), proton pump inhibitors (for gastroesophageal reflux disease), and antihypertensive medications (for renal crisis). However, there are no therapies that effectively reverse or halt the underlying disease process, typified by fibrosis and vasculopathy.
Notable Drugs:
- Nintedanib, an antifibrotic agent recently approved for SSc-associated interstitial lung disease (ILD), and tocilizumab, an IL-6 receptor blocker, are among the recent noteworthy additions to the SSc treatment arsenal. They signify the move towards more targeted therapies for SSc.
Unmet Medical Need:
Despite these advancements, significant unmet needs in SSc treatment remain. No therapy has yet proven effective across all manifestations of the disease, particularly in modifying the underlying disease process and improving long-term outcomes. The morbidity and impact on quality of life are high, and mortality rates, particularly in patients with pulmonary involvement, remain concerning.
Market Opportunity for KYV-101:
Based on its mode of action, KYV-101 could address a key component of SSc pathology – the role of B cells in autoimmunity. By potentially resetting the immune system, KYV-101 could offer a more definitive intervention than is currently available, assuming it proves to be effective in removing pathogenic B cells and shows favorable safety and tolerability profiles.
Given the estimated prevalence of 200,000 diagnosed SSc patients in the US, EU, and Japan, and the likelihood that a significant proportion of these patients have disease refractory to current treatments, KYV-101 may fill a crucial niche in the treatment armamentarium. Furthermore, the high costs associated with managing chronic complications of SSc represent an economic burden that an effective therapy could alleviate, potentially justifying a high-value market proposition for a successful treatment.
However, to capitalize on this opportunity, demonstration of conclusive clinical benefits in robust trials will be crucial. Efficacy in improving or stabilizing fibrosis, preventing organ failure, and overall survival benefits would represent significant clinical advancements. Moreover, the drug's impact on quality of life and long-term health economics will be factors in its market positioning.
Competition and Market Penetration:
Any new entrant to the SSc treatment market, including KYV-101, will have to navigate an environment with established and emerging therapies. Differentiation in terms of efficacy, safety, and cost-effectiveness will be key to gaining market share. Given the rarity of SSc, there may also be opportunities for orphan drug status and incentives, which can facilitate drug development and market exclusivity.
In summary, the CAR T-cell therapy KYV-101 has the potential to meet a high unmet need in SSc treatment if it can demonstrate efficacy in reducing or reversing autoimmune-driven tissue damage while proving to be safe and manageable for chronic use. Strategic development, comprehensive clinical data, and navigating the complex regulatory and reimbursement landscapes will be critical for success in this market.
Competitors for KYV-101:
Given the intense competition in the biopharmaceutical industry and the continuous efforts to discover and develop new treatments for autoimmune diseases, including systemic sclerosis (SSc), KYV-101 may face significant competition from a variety of therapeutic approaches.
Biologics and Monoclonal Antibodies:
- Rituximab (Rituxan): An anti-CD20 monoclonal antibody used off-label for SSc and other autoimmune diseases, which depletes B-cells.
- Ocrelizumab (Ocrevus): Another anti-CD20 monoclonal antibody that has gained attention for its use in multiple sclerosis and could be considered for other B-cell-related autoimmune conditions.
Small Molecule Inhibitors:
New oral medications that target specific pathways implicated in fibrosis or inflammation are also being developed. These drugs offer the advantage of oral administration compared to the intravenous or subcutaneous delivery required for many biologics and CAR T-cell therapies.
Stem Cell Transplantation:
Autologous hematopoietic stem cell transplantation is a treatment option for severe, rapidly progressing SSc. This aggressive treatment can offer significant improvement for some patients but comes with the risk of serious side effects, including treatment-related mortality.
Competitive Landscape:
Emerging treatments from large pharmaceutical companies as well as smaller biotech firms are likely to offer competitive challenges for KYV-101. The safety profiles, dosing convenience, and potential long-term benefits are key factors in this competition.
The proprietary nature of KYV-101 as an investigational CAR T-cell therapy does offer a novel approach, potentially providing significant efficacy in modifying the disease course; however, this will need to be balanced against the invasiveness and potential risks of the therapy, as well as the costs associated with CAR T-cell treatments, which can be substantial.
Success in the competitive landscape of systemic sclerosis treatments will be heavily reliant on KYV-101's clinical efficacy in trial outcomes, its safety and tolerability, and the overall feasibility of treatment in terms of patient management. It will also depend on regulatory approval, reimbursement mechanisms, and market positioning in terms of treatment sequencing—whether KYV-101 is considered as a first-line therapy, a rescue treatment, or a last resort for refractory cases.
Considering that systemic sclerosis is a complex and multifactorial disease, combination therapies may also emerge as a competitive strategy, potentially integrating treatments like KYV-101 with other immunomodulators or antifibrotics.
Ultimately, a thorough understanding of the evolving treatment ecosystem and the real-world impacts of the emerging therapies—including KYV-101—on patients' quality of life and long-term outcomes will be crucial for determining the potential success of these agents in the SSc market.
The treatment of systemic sclerosis (SSc) focuses largely on symptom management and the treatment of specific organ involvement due to the lack of a cure. However, several notable medications have been traditionally used or more recently approved to address various aspects of the disease.
Traditional Treatments for Systemic Sclerosis:
- Immunosuppressants: Drugs like cyclophosphamide and mycophenolate mofetil are used, particularly for pulmonary involvement, to suppress the immune system and potentially reduce fibrosis.
- Corticosteroids: These are used for their anti-inflammatory effects in skin and musculoskeletal manifestations.
- Vasodilators: For instance, calcium channel blockers are used to treat Raynaud's phenomenon in SSc patients, which can help prevent ulcers and improve blood flow to extremities.
- Proton pump inhibitors (PPIs): Used to reduce acid reflux symptoms and prevent damage to the esophagus.
- Angiotensin-converting enzyme (ACE) inhibitors: Used in cases of SSc renal crisis for their antihypertensive effects.
Recently Approved Drugs:
- Nintedanib (Ofev): This is an antifibrotic agent originally approved for idiopathic pulmonary fibrosis (IPF) and subsequently received FDA approval in 2019 for use in patients with SSc-associated interstitial lung disease (ILD). Nintedanib slows down the rate of decline in lung function.
- Tocilizumab (Actemra): An IL-6 receptor inhibitor that was approved in some countries for the treatment of systemic sclerosis. While not approved in the United States for this indication, it has shown some efficacy in reducing the rate of progression of lung disease in SSc-ILD.
Investigational Therapies:
There are several investigational drugs in the pipeline for treating SSc, including various targeted biologics and small molecules that aim to reduce inflammation and fibrosis, as well as improve vascular symptoms. These potential future treatments, depending on their success in clinical trials, may offer new hopes for SSc patients.
Treatment Modalities:
In addition to pharmacological interventions, treatment modalities for SSc may include physical therapy to maintain mobility, occupational therapy, and interventions to manage gastrointestinal and other complications.
In the future, treatments like KYV-101 could potentially play a role, especially for refractory cases of SSc, if they prove to be successful in clinical trials. The ability to reset the immune system by targeting autoreactive B cells would create a novel treatment pathway separate from the immunosuppressants and symptom management approaches currently used.
As the understanding of SSc advances, it is hoped that more effective and targeted treatments will become available, thereby improving the prognosis and quality of life for those affected by this multifaceted disease.
KYV-101, a CAR T-cell therapy targeting CD19, is showing promise in its initial clinical trials for the treatment of lupus nephritis (LN), a similar autoimmune disease to systemic sclerosis (SSc).
Given that KYV-101 is designed to deplete B-cells and thereby potentially modulate the autoimmune response, its application to SSc might fit within the broader treatment landscape as follows:
- Add-on to Standard Care: KYV-101 might be considered as an add-on to the existing standard-of-care treatments for SSc, particularly for patients with refractory disease who have had an inadequate response to conventional therapies.
- Therapeutic Alternatives for Severe Cases: As SSc can have life-threatening complications, particularly SSc-ILD and severe vascular manifestations, the immunomodulatory effects of KYV-101 could be valuable as a novel approach to treating severe or rapidly progressing cases.
- B-cell Targeted Therapy: Building on the similar rationale to B-cell depleting therapies like rituximab (used off-label), KYV-101 could be positioned as an advanced therapy - assuming clinical trial success - for patients who are not adequately managed with existing biologics or who require a more profound and durable B-cell depletion.
Despite potential benefits, certain considerations must be accounted for regarding the introduction of KYV-101 into the standard of care for SSc:
- Safety Profile: As outlined in the development of KYV-101 for LN, safety and tolerability are crucial, especially when considering CAR T-cell therapies for chronic diseases. Given that serious CRS and ICANS have not been observed with KYV-101, if this safety profile holds in larger trials, it could be a major factor in its acceptance for treating SSc.
- Ease of Use: Unlike oncological settings where CAR T-cell therapies are often used as a last resort for life-threatening conditions, for chronic diseases like SSc, the ease of treatment administration, monitoring, and patient quality of life post-therapy will significantly impact its place in the standard of care.
- Efficacy: The therapy must demonstrate clear benefits in improving disease outcomes, such as skin thickening and organ function, which are critical issues for SSc patients.
- Cost-effectiveness: CAR T-cell therapies are known for being expensive, which could potentially limit access and acceptance unless they demonstrate cost-effectiveness through improved disease outcomes and reduced long-term healthcare costs.
Overall, should further clinical development demonstrate significant efficacy and an acceptable safety profile, KYV-101 might offer a novel treatment avenue for patients with SSc, potentially fitting into the standard care as a targeted therapy for severe, refractory, or B-cell-driven subsets of the disease. Its ultimate place in treatment algorithms will depend on a variety of factors including clinical trial outcomes, regulatory approvals, cost, accessibility, patient and physician preference, and evolving standards of care.
Myasthenia gravis
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder characterized by weakness and fatigue of the skeletal muscles. It occurs when the normal communication between nerves and muscles is interrupted at the neuromuscular junction – the place where nerve cells connect with the muscles they control.
Pathology:
The pathology of MG is primarily attributed to an immune-mediated response where antibodies incorrectly target and damage the proteins involved in the neuromuscular transmission. The most common target of these antibodies is the acetylcholine receptor (AChR) at the neuromuscular junction. Acetylcholine (ACh) is a neurotransmitter that carries signals from nerve endings to the muscle receptors. In MG, antibodies block, alter, or destroy the receptors for ACh at the neuromuscular junction, which prevents the muscle contraction from occurring, thus causing muscle weakness.
In addition to AChR antibodies, some individuals with MG have antibodies against a muscle-specific tyrosine kinase (MuSK), which plays a role in the formation and maintenance of the neuromuscular junction, or other less common targets such as lipoprotein-related protein 4 (LRP4) and agrin.
Symptoms:
- Ocular Muscle Weakness: Drooping of one or both eyelids (ptosis) and double vision (diplopia), which are often the first signs of MG.
- Bulbar Muscle Weakness: Difficulty in swallowing, speaking, and chewing due to weakness of the muscles controlling these functions.
- Facial Muscle Weakness: Altered facial expressions and difficulty in smiling or closing eyes tightly.
- Limb Muscle Weakness: Weakness in arms and legs which can affect mobility and performing daily activities.
- Respiratory Muscle Weakness: In severe cases, MG can cause respiratory failure due to the muscles used for breathing becoming too weak, which is a medical emergency known as a myasthenic crisis.
Prognosis:
While MG can affect individuals of any age, it most commonly presents in women under 40 and men over 60. The prognosis for individuals with MG has significantly improved over time, thanks to advancements in treatments. Most people with MG can expect to live a normal or near-normal lifespan. However, they may require ongoing treatment to maintain muscle strength and function. Some patients experience periods of remission, where treatment may not be necessary.
Diagnosis:
Diagnosing MG may involve a combination of various tests, including the detection of AChR antibodies, electrophysiological tests like repetitive nerve stimulation (RNS) and single-fiber electromyography (SFEMG), and clinical tests such as the edrophonium (Tensilon) test, which can briefly improve muscle strength in MG patients due to increased levels of ACh at the neuromuscular junction.
Treatment:
- Cholinesterase Inhibitors: Drugs like pyridostigmine are used to increase the concentration of ACh at the neuromuscular junction.
- Immunosuppressants: Medications including corticosteroids and azathioprine are typically used to suppress the immune response.
- Plasmapheresis and Intravenous Immunoglobulin (IVIg): These are used for rapid, short-term symptom improvement, particularly in myasthenic crisis or in preparation for surgery.
- Monoclonal Antibodies: Eculizumab, a drug that inhibits the complement cascade, has shown efficacy in AChR antibody-positive MG.
- Thymectomy: The surgical removal of the thymus gland, which is thought to play a role in the abnormal immune response, is beneficial for some MG patients, especially those with thymomas.
In summary, MG is a chronic condition that can be managed effectively with treatment protocols tailored to the severity of individual symptoms and the response to therapy. With appropriate management, patients can lead active lives, although vigilance is required for potential exacerbations, including myasthenic crisis.
KYV-101, a CAR T-cell therapy targeting CD19, could represent a novel therapeutic option for myasthenia gravis (MG), particularly in patients who have not responded to existing treatments or cannot tolerate them. CAR T-cell therapies like KYV-101 are designed to target and deplete B cells, which are responsible for the production of the pathogenic autoantibodies that disrupt neuromuscular transmission in MG.
Standard of Care for Myasthenia Gravis:
The current standard of care for MG includes cholinesterase inhibitors such as pyridostigmine to improve muscle contractions, immunosuppressive drugs to reduce antibody production (e.g., corticosteroids, azathioprine, and mycophenolate mofetil), and thymectomy in cases where thymoma or thymic hyperplasia is present. In patients with severe MG or in those experiencing a myasthenic crisis, treatments such as intravenous immunoglobulin (IVIg) and plasmapheresis are used to rapidly remove circulating autoantibodies.
Recently Approved Drugs:
Eculizumab (Soliris) is a complement inhibitor that has been approved for the treatment of AChR antibody-positive MG, offering benefits to patients by inhibiting the complement pathway, which is involved in the destruction of the neuromuscular junction in MG.
Unmet Medical Need:
There is a substantial unmet need in MG for treatments that offer durable remission and address refractory disease. Some patients have MG that is refractory to standard therapies or experience intolerable side effects. Furthermore, the treatments do not provide a cure and are instead aimed at managing symptoms, which means patients can experience fluctuations in disease severity and quality of life. As MG is a chronic condition requiring long-term management, there is also a need for treatments that can reduce the burden of continuous medication and associated side effects.
Market Opportunity for KYV-101:
The market opportunity for KYV-101 in MG is anchored by the prevalence of the disease, which includes around 160,000 diagnosed patients across the US, EU, and Japan. Additionally, if a significant portion of MG patients are refractory or intolerant to existing therapies, KYV-101 could provide a much-needed therapeutic option for this population.
CAR T-cell therapies have the potential to offer a one-time treatment that may confer sustained periods of remission by reducing or eliminating the autoreactive B cells that underlie the pathogenesis of MG. The attractiveness of KYV-101 in the MG treatment market will depend on its efficacy in providing long-term symptom control without continual medication and its safety profile compared to the lifelong immunosuppression that is the current standard of treatment.
Conclusion and Anticipated Challenges:
The success of KYV-101 in the MG market will depend on several factors:
- Clinical Efficacy: Demonstrating significant improvement in muscle weakness and reductions in the need for other immunosuppressive medications.
- Safety: Showing a favorable side effect profile, given CAR T-cell therapy can be associated with severe side effects, including CRS and ICANS, which although not observed in initial MS patients treated with KYV-101, could be a concern.
- Cost and Accessibility: CAR T-cell therapies are expensive, and affordability and insurance coverage may play a role in the adoption of KYV-101 if approved.
- Durability: Providing long-lasting remission and reducing the need for chronic treatment would be a key differentiator for KYV-101.
The aforementioned challenges align with broader considerations in the adoption of new therapies for chronic autoimmune diseases: the balance between benefit, risk, and resource allocation. If KYV-101 can successfully address these challenges in clinical trials, the therapy could become an important addition to the MG treatment landscape, catering to a population of patients with disease refractory to current therapies or those seeking a more sustainable long-term treatment option.
In the treatment landscape for Myasthenia Gravis (MG), there are several promising therapies under development that could potentially compete with KYV-101. These emerging treatments are focused on various aspects of the disease's underlying mechanisms and strive to improve upon current standards of care by enhancing efficacy, reducing side effects, and offering easier routes of administration. Here are some notable examples:
Monoclonal Antibodies:
- Efgartigimod (Argenx): An FcRn antagonist designed to reduce the levels of pathogenic IgG antibodies, including AChR antibodies.
- Rituximab (Rituxan): While already used in an off-label capacity for MG, there are ongoing studies to confirm its efficacy and safety in this specific condition. As an anti-CD20 monoclonal antibody, it depletes B cells, similar to the action of KYV-101.
Complement Inhibitors:
- Eculizumab (Soliris): A C5 complement inhibitor already approved for use in AChR antibody-positive MG, this drug prevents the formation of the membrane attack complex (MAC) that can damage the neuromuscular junction.
- Ravulizumab (Ultomiris): A next-generation complement C5 inhibitor with a longer duration of action compared to eculizumab, potentially allowing for less frequent dosing.
- Zilucoplan and Pozelimab: Small molecule inhibitors of complement C5 being investigated for their benefit in MG.
T-Cell Modulators:
- Amifampridine (Firdapse): Approved for Lambert-Eaton myasthenic syndrome (LEMS) and MG, this drug increases the release of ACh from nerve endings to enhance neuromuscular transmission.
B-Cell Modulators:
- Belimumab (Benlysta): An inhibitor of B lymphocyte stimulator (BLyS), is being investigated in clinical trials to assess its efficacy in treating MG.
Stem Cell Transplant Therapies:
Autologous hematopoietic stem cell transplantation (HSCT) is being researched as a potential intervention for severe, treatment-refractory MG, aiming to 'reset' the immune system by replacing it with self-derived, immunologically naive stem cells.
These therapies offer a direct comparison to the mechanism of action of KYV-101. The key factors that will define competitiveness in this space will likely be the safety and efficacy profiles demonstrated in clinical trials, the convenience and acceptability of treatment regimens to patients, cost, and the context within which therapies are used (first-line treatment, refractory disease, etc.).
Moreover, advances in biologics, such as developing subcutaneous formulations, as opposed to intravenous ones, hold promise for increasing patient compliance and convenience.
Another competitive factor will be the regulatory milestones achieved by these therapies, such as breakthrough therapy designation, accelerated approval, or orphan drug status, which can facilitate a more rapid path to market.
As the treatment landscape for MG evolves, KYV-101 must demonstrate a clear benefit in managing MG symptoms, reducing the need for chronic immunosuppression, and providing a desirable safety profile to carve out a significant market share. Its success will also depend on the ability to effectively position it within the treatment algorithm for MG, whether as a line of therapy for newly diagnosed patients, a treatment for those who have been refractory to other interventions, or as a maintenance therapy for long-term disease control.
Myasthenia gravis (MG) is managed by various treatments aimed at diminishing the autoimmune response or improving neuromuscular transmission. Notable drugs used in MG management include both time-tested options and recently approved therapies:
Traditional Treatments:
- Cholinesterase Inhibitors: Pyridostigmine bromide (Mestinon) is commonly used to increase levels of acetylcholine at the neuromuscular junction, enhancing communication between nerves and muscles.
- Corticosteroids: Prednisone is frequently used for its immunosuppressive effects that can reduce antibody production.
- Immunosuppressants: Azathioprine (Imuran), mycophenolate mofetil (CellCept), and cyclosporine are used to modify patients' immune systems long-term.
Intravenous Treatments:
- Intravenous Immunoglobulin (IVIg): Provides a transient modulation of the immune system and can be used in managing exacerbations or as a maintenance therapy.
- Plasmapheresis (Plasma Exchange): Used to rapidly remove circulating antibodies, often during a myasthenic crisis or pre-operatively.
Recently Approved Drugs:
- Eculizumab (Soliris): Approved by the FDA in 2017 for the treatment of adults with generalized MG who are anti-acetylcholine receptor (AChR) antibody-positive. Eculizumab is a monoclonal antibody that inhibits the complement system, which is thought to play a role in the destruction of the neuromuscular junction in MG.
- Efgartigimod (Vyvgart): Approved by the FDA in December 2021, efgartigimod is a neonatal Fc receptor (FcRn) antagonist designed to reduce the levels of pathogenic IgG antibodies.
- Amifampridine (Firdapse/Ruzurgi): Approved for Lambert-Eaton myasthenic syndrome (LEMS), amifampridine has also been used off-label for MG because it increases the release of acetylcholine from nerve endings, enhancing neuromuscular transmission.
Surgical Intervention:
- Thymectomy: Surgical removal of the thymus gland (which is often abnormal in MG patients) is indicated for those with thymomas and has demonstrated benefit even in non-thymomatous MG.
Investigational Treatments:
There are ongoing clinical trials evaluating new therapeutic agents for MG, including additional monoclonal antibodies targeting different components of the immune system, small molecule drugs, and other innovative immunotherapies.
The entry of new treatments, like the aforementioned efgartigimod (Vyvgart), reflects the need for more targeted and effective management strategies for MG. For a new therapy like KYV-101 to be successful, it will need to demonstrate at least comparative efficacy to these established and newly approved treatments, with an acceptable safety and tolerability profile. Moreover, given the chronic nature of MG, ease of administration, impact on quality of life, and cost of therapy are also crucial factors that will influence adoption and use in clinical practice.
The implications of its application to myasthenia gravis (MG) offer interesting possibilities for incorporation into the standard of care for MG, given the commonality of B-cell involvement in the pathogenesis of these autoimmune diseases.
Here are a few potential roles that KYV-101 might assume within the MG treatment paradigm:
Refractory Myasthenia Gravis:
For patients who have not responded to standard therapies, such as cholinesterase inhibitors, corticosteroids, and other immunosuppressants, or who experience significant side effects from these treatments, KYV-101 could offer an alternative option. Successful outcomes in the lupus nephritis trials indicating an improvement in proteinuria without significant adverse events, such as serious cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS), may suggest similar potential in MG for effectively reducing pathogenic autoantibodies.
Potential as a 'Reset' Therapy:
The ability of CAR T-cell therapies to provide a "reset" to the immune system could resonate well in the context of MG, where long-term immunomodulation is often required. If KYV-101 can lead to sustained remission by effectively depleting the B cells that produce the acetylcholine receptor antibodies, this could represent a major advancement for patients, particularly those with antibody-positive MG.
Safety and Tolerability:
The safety profile of KYV-101 will be critical in determining its suitability for MG patients. The lack of serious CRS and ICANS in early trials is promising. However, the application of CAR T-cell therapies in chronic autoimmune conditions, where long-term safety is a significant concern, will require comprehensive safety evaluation.
Considerations for Integration into Standard of Care:
- It would need to demonstrate efficacy in reducing muscle weakness and other MG symptoms.
- It would need to show a safety and tolerability profile that is at least as good as, if not better than, current treatment options.
- There must be careful consideration of the cost-benefit analysis, as CAR T-cell therapies tend to be expensive.
- It would require approval from regulatory agencies, which would be predicated on evidence of effectiveness and safety from clinical trials.
KYV-101, if proven effective for MG, could represent a shift towards more targeted and durable treatments for autoimmune diseases. However, its role may initially be confined to a subset of patients with severe, refractory MG due to the novel nature of the therapy and the considerations around administering a CAR T-cell treatment, such as the need for specialized healthcare facilities.
Market adoption would depend on the data emerging from trials, which would need to conclusively demonstrate an improvement in clinical outcomes for MG patients. Moreover, the logistics of treatment, including the process of cell collection, modification, and reinfusion, and patient monitoring post-infusion, would need to be streamlined for widespread use. Overall, the positioning of KYV-101 within the MG treatment landscape will be a function of efficacy, safety, practicality, and economics as compared to established and emerging therapies.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic, progressive autoimmune disease that affects the central nervous system (CNS), which includes the brain and spinal cord. It is characterized by the immune system's attack on the protective covering of nerve fibers known as the myelin sheath, causing inflammation and consequent damage. This damage impairs the signaling and communication between the brain and various parts of the body.
The exact cause of MS is unknown; however, it's believed to be a result of a combination of genetic susceptibility and environmental factors such as viral infections, smoking, and vitamin D deficiency. The disease process is marked by:
- Demyelination: The immune system incorrectly targets the myelin, leading to patches of demyelinated tissue, or lesions. This disrupts the efficient transmission of electrical impulses along nerve fibers.
- Inflammation: Both the myelin and the underlying nerve fiber can be damaged or destroyed in active lesions, which are typically associated with the presence of inflammatory cells.
- Axonal and neuronal damage: As the disease progresses, loss of axons and neurons can occur, which contributes to permanent neurological disability.
- Formation of scar tissue (sclerosis): The body tries to repair the damage caused by inflammation, but this can lead to scarring or sclerosis, which gives the disease its name.
MS symptoms vary widely and depend on the amount of nerve damage and which nerves are affected. Common symptoms include:
- Fatigue
- Numbness and tingling
- Muscle weakness and spasticity
- Visual disturbances
- Dizziness and vertigo
- Cognitive issues
- Emotional changes and depression
- Autonomic dysfunction
- Sensory disturbances
- Sexual dysfunction
MS is unpredictable, and its course varies. Most people with MS have a relapsing-remitting disease course characterized by episodes of new or increasing neurologic symptoms followed by periods of partial or complete recovery. A subset of individuals eventually transition to a secondary progressive course, where there is a gradual worsening of symptoms over time with or without relapses. Another form is primary progressive MS, where there is a steady progression of the disease from onset without relapses.
While MS is not curable, treatments can help control symptoms and modify the disease course. Advances in disease-modifying therapies have significantly improved the outlook for many patients, and while life expectancy may be slightly reduced, many with MS can manage symptoms effectively and maintain a good quality of life with treatment.
Diagnosis of MS requires a combination of medical history, clinical examination, and supporting data from imaging, lab tests, and electrophysiological tests to rule out other causes and confirm MS.
Treatment strategies for MS include:
- Disease-modifying therapies (DMTs): Drugs that reduce the frequency and severity of relapses and slow the progression of the disease.
- Symptomatic treatments: For managing symptoms like pain, spasticity, fatigue, and bladder issues.
- Rehabilitation therapies: Including physical, occupational, and speech therapy.
- Lifestyle changes: Including regular exercise, a healthy diet, and smoking cessation.
In summary, MS is a complex disease that can lead to significant physical and cognitive impairment, but with the advancements in therapeutic options, patients are experiencing improved clinical outcomes and quality of life. However, since MS varies from person to person, its management is highly individualized.
KYV-101, a CD19-targeting CAR T-cell therapy, represents an innovative approach to the treatment of multiple sclerosis (MS), a disease for which there is considerable unmet medical need, despite the availability of numerous disease-modifying therapies (DMTs).
Standard of Care:
The current standard of care for MS includes a variety of DMTs that cater to different patient profiles and disease severities:
- Injectable therapies: This includes interferon beta-1a and beta-1b, and glatiramer acetate, which are often used as first-line treatments.
- Oral DMTs: Fingolimod, teriflunomide, and dimethyl fumarate are commonly prescribed second-line options.
- High-efficacy infusion therapies: Natalizumab, alemtuzumab, and ocrelizumab are usually reserved for more active forms of the disease or when patients fail to respond to first-line DMTs.
Successful Drugs and Developments:
Ocrelizumab (Ocrevus) marks a significant advancement in MS treatments as the first therapy approved for both relapsing forms of MS and primary progressive MS, highlighting the crucial role of B cells in disease pathogenesis. It's been evidenced to reduce the frequency of relapses and slow disease progression.
Unmet Medical Need:
The heterogeneity and the progressive nature of MS mean that many patients do not respond optimally to existing treatments. Some of the common challenges include:
- Incomplete control of disease progression, particularly in primary progressive MS.
- Side effects and safety concerns of existing DMTs leading to treatment discontinuation.
- The need for treatments that provide neuroprotective or remyelinating effects, which current therapies do not offer.
- A growing patient population seeking therapies that are more tolerable and convenient, and that offer improved quality of life.
Market Opportunity for KYV-101:
Given this backdrop, KYV-101 could potentially carve a niche for itself by addressing several of these unmet needs:
- Resetting Immune System: The potential of KYV-101 to produce a more profound and lasting B-cell depletion than existing therapies like ocrelizumab could offer a more effective way of halting disease progression.
- Deep Tissue Penetration: If KYV-101 can better penetrate CNS tissues and dismantle the autoimmune process at the site of pathology, it might provide advantages over treatments that work peripherally.
- One-Time Treatment Potential: CAR T-cell therapies have the promise of a one-time treatment leading to long-term remission, which could be particularly appealing in the MS market where lifelong treatment is currently the norm.
- Safety Profile: The incidence of no ICANS and only one patient experiencing Grade 1 CRS reported during the clinical development for KYV-101 in MS is encouraging. A favorable safety profile is essential for its acceptance.
Challenges and Considerations:
KYV-101 will face considerable scrutiny because MS is a life-long disease requiring chronic treatment. The potential long-term effects of a one-off CAR T-cell therapy in a non-lethal disease, the logistics of cell-based therapy, reimbursement, and cost of treatment are important considerations in its path to the market. Additionally, the potential to offer KYV-101 as a remission-inducing therapy for those patients not responding to other DMTs could be a key positioning strategy.
It will also compete with increasingly effective DMTs, some of which are exploring less frequent dosing schedules and improved safety profiles. Furthermore, several remyelinating and neuroprotective therapies are currently in development, aiming to repair the damage caused by MS rather than just modulate the immune system.
In conclusion, KYV-101's potential to reset the immune system and offer a sustained response could meet a significant unmet need for MS patients, particularly those with aggressive disease forms, provided it continues to demonstrate strong clinical efficacy and a manageable safety profile in broader patient populations.
In the field of multiple sclerosis (MS) treatment, numerous therapies are in various stages of development that have the potential to compete with KYV-101, the CD19-targeting CAR T-cell therapy. Given the complex etiology of MS and the varying response of patients to treatments, there is a robust pipeline of potential competitors targeting different aspects of the disease's pathophysiology.
Biologics and Monoclonal Antibodies:
- Ocrelizumab (Ocrevus): This anti-CD20 B-cell depleting agent is already approved for relapsing and primary progressive MS and has set a precedent for similar B-cell targeting strategies like KYV-101.
- Ofatumumab (Kesimpta): Another anti-CD20 monoclonal antibody designed for subcutaneous administration, offering convenience compared to intravenous infusion.
- Ublituximab: An investigational glycoengineered monoclonal antibody targeting CD20, which is in the late stages of clinical development.
S1P Receptor Modulators:
Newer sphingosine 1-phosphate (S1P) receptor modulators are being developed to improve the benefit-risk profile compared to the first-generation S1P receptor modulators such as fingolimod. Examples include Ozanimod (Zeposia), Ponesimod (Ponvory), and Siponimod (Mayzent), which is specifically approved for secondary progressive MS.
Anti-LINGO-1 and Remyelinating Therapies:
These focus on promoting remyelination and neuroprotection, which could be viewed as complementary or supplementary to the therapeutic strategies aiming at immunomodulation. An example is Opicinumab (Anti-LINGO-1), intended to promote remyelination and preserve axonal integrity.
Tolerogenic Vaccines and Antigen-Specific Approaches:
Innovative strategies to induce immune tolerance to MS-related antigens are being explored. These aim to retrain the immune system to prevent the attacks on myelin.
Cell-Based Therapies:
- Autologous Hematopoietic Stem Cell Transplant (AHSCT): Although not proprietary, AHSCT is conducted in specialized centers and has shown efficacy in completely resetting the immune system in select aggressive cases of MS with the hopes of achieving long-term remission.
Neuroprotective Agents:
Drugs that protect neuronal integrity and prevent neurodegeneration are being researched, although none have yet gained approval for MS.
KYV-101 will need to demonstrate a favorable efficacy and safety profile to effectively compete, especially considering the risks associated with CAR T-cell therapies relative to lower-risk DMTs. For example, the success of anti-CD20 therapies like ocrelizumab offers proof-of-concept for B-cell targeting in MS, but KYV-101 will need to provide additional benefits, such as greater efficacy or longer duration of remission, to be competitive.
Furthermore, CAR T-cell treatment in MS would represent a significant shift from the current standard of care. Issues such as cost, complexity of administration, management of potential side effects like cytokine release syndrome (CRS), and the long-term safety considerations in a non-lethal but chronic disease will all be factors influencing the acceptance and adoption of such therapies.
While KYV-101 may fulfill a niche for patients with highly active, treatment-refractory MS or those who prefer a potentially one-time treatment over continuous treatment, its broader adoption would depend on the balance of these benefits and risks, along with the economic viability for healthcare systems and patients.
Multiple sclerosis (MS) is a complex disease with a wide range of treatment options. Several notable drugs have been approved for MS treatment, ranging from older, well-established therapies to newly approved drugs that represent advancements in the field. Here is a list highlighting some key treatments:
Injectable Treatments:
- Interferon beta-1a and beta-1b: These include Avonex, Betaseron, Rebif, and Plegridy. They are among the first disease-modifying therapies (DMTs) and work by modulating the immune system to reduce inflammation.
- Glatiramer acetate (Copaxone): This is a synthetic protein that simulates myelin basic protein and serves as a decoy to divert the immune attack away from the myelin sheath.
Oral Treatments:
- Fingolimod (Gilenya): The first oral DMT for MS, which acts as a sphingosine 1-phosphate (S1P) receptor modulator, sequesters lymphocytes in lymph nodes, preventing them from reaching the CNS.
- Dimethyl fumarate (Tecfidera): An oral agent which is believed to have anti-inflammatory and neuroprotective effects.
- Teriflunomide (Aubagio): An oral pyrimidine synthesis inhibitor with anti-inflammatory properties.
Monoclonal Antibodies:
- Natalizumab (Tysabri): An infusion therapy that prevents immune cells from crossing the blood-brain barrier and causing inflammation and damage in the CNS.
- Ocrelizumab (Ocrevus): Approved in 2017, it's the first treatment for both relapsing-remitting MS (RRMS) and primary progressive MS (PPMS). It depletes CD20-positive B cells, which are implicated in the MS disease process.
- Alemtuzumab (Lemtrada): Targets CD52, leading to the destruction of both B and T cells. It is reserved for individuals with severe disease due to potential risks.
Recently Approved Drugs:
- Siponimod (Mayzent): Approved in 2019, is an S1P receptor modulator for the treatment of SPMS, as well as clinically isolated syndrome (CIS) and RRMS.
- Cladribine (Mavenclad): Approved in 2019, is an oral drug that selectively targets lymphocytes thought to be involved in the pathogenesis of MS.
- Ofatumumab (Kesimpta): Approved in 2020, is a self-administered, subcutaneous anti-CD20 monoclonal antibody for RRMS.
- Ponesimod (Ponvory): Approved in 2021, another S1P receptor modulator, given orally once daily for the treatment of adults with RRMS.
- Ozanimod (Zeposia): Approved in 2020, is an oral S1P receptor modulator similar to fingolimod, potentially with a better safety profile.
These treatments have changed the landscape of MS management by providing a variety of options for patients and clinicians. The right choice of therapy depends on individual patient factors, including the subtype of MS, disease activity level, safety considerations, and patient preferences. Continuing research and development bring hope for further advancements in this field, which could impact KYV-101's position in the market if approved for the treatment of MS.
While the detailed company information provided does not address KYV-101 in the specific context of multiple sclerosis (MS), similar principles may apply given that both lupus nephritis and MS are B cell-mediated autoimmune diseases. Based on the successes seen with KYV-101 in lupus nephritis, one might extrapolate potential applications for MS. Here's how KYV-101 might fit into the standard of care for MS:
Potential Role in MS Treatment:
Even with numerous existing disease-modifying therapies (DMTs) for MS, there remain significant unmet needs for a subset of patients, particularly around improving long-term outcomes and managing progressive forms of the disease. KYV-101, as a CAR T-cell therapy targeting B cells, could potentially fulfill the following roles:
- Addressing Refractory MS: For patients with highly active, relapsing-remitting MS that does not respond to conventional DMTs, KYV-101 may offer an alternative by providing deeper B cell depletion and sustained remission. This could be particularly relevant for individuals who have not benefited from other B cell depleting agents like ocrelizumab.
- Treating Progressive MS: In primary and secondary progressive MS, where options are limited and the disease continues to advance, KYV-101 might be considered if it can show effectiveness in stopping or slowing down the progression.
- Improving Compliance and Reducing Treatment Burden: If KYV-101 can offer long-lasting effects with a single treatment, it could improve patient compliance and quality of life compared to long-term DMT regimens, which often involve ongoing drug administration and associated side effects.
Safety and Efficacy Concerns:
MS treatments must have a favorable risk-benefit ratio, given the chronic nature of the disease and the patient population, which generally retains a relatively high level of function. The absence of serious cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) in the KYV-101 trial with lupus nephritis patients is promising. However, careful consideration must be given to long-term safety, particularly when applying a one-time treatment with permanent effects, like CAR T-cell therapy, in a non-lethal disease such as MS.
Market Penetration:
KYV-101's potential market entry will depend on further clinical development, including the success of phase 2/3 trials demonstrating its efficacy and safety in MS. Given that MS treatment is often lifelong with a substantial financial burden, the cost of KYV-101 will also be a significant factor for market penetration, particularly when compared to existing first-line treatments.
Conclusion:
KYV-101 could fit into the standard of care for MS, particularly for patients who are refractory to existing therapies or for whom those therapies are contraindicated or produce undesirable side effects. The degree to which it is incorporated will depend on a multitude of factors including, but not limited to, the demonstration of clear clinical benefit, manageable safety profile, reimbursement pathways, and treatment accessibility. As the MS treatment landscape evolves with the introduction of more DMTs offering various modes of action, regimens, and potencies, KYV-101's differentiation will hinge on offering durable and profound efficacy along with satisfactory patient safety and quality of life.
Financial modeling
Lupus nephritis
I will create a hypothetical revenue build with placeholder estimates. Please note that actual revenue generation could significantly differ as it depends on numerous factors, including trial outcomes, commercial strategies, market conditions, payer negotiations, and competitor dynamics.
Hypothetical Revenue Build for KYV-101 in Lupus Nephritis
- Target Population Size (U.S. and Germany): For the sake of this analysis, let's assume a target population of 100,000 LN patients in the U.S. and Germany combined.
- Penetration Rate / Access to Treatment:
- Year 1 Post-Approval: 0.5% penetration = 500 patients
- Year 2 Post-Approval: 1% penetration = 1,000 patients
- Year 3 Post-Approval: 2% penetration = 2,000 patients
- Insurance Coverage: Assume that 80% of patients have insurance coverage and 20% may receive drug access through patient assistance programs at a reduced cost or free, affecting the revenue.
- Duration of Therapy:
- Average treatment duration of 12 months per patient, with the assumption that patients will be on continuous treatment for the year.
- Market Growth Assumption: We could assume the target patient population or penetration rate may grow at a compound annual growth rate (CAGR) of 5-10% based on increased disease prevalence, diagnosis rates, and improved access to treatment.
- Impact of Competition and Payer Negotiation: Additional competitors entering the market or aggressive payer negotiations could decrease the net revenue. This should be modeled as a potential downside risk in the revenue forecast.
Remember that these numbers are entirely hypothetical and a range of scenarios should be modeled considering best-case, base-case, and worst-case sales forecasts. The projections assume prompt regulatory approval, success in manufacturing and commercialization, and a favorable reimbursement landscape. Adjustments should be considered based on real-world data, evolving competitive space, and changing market dynamics. Moreover, getting input from market research, pricing and reimbursement specialists, and forecasting consultants might refine these numbers further.
Systemic sclerosis
To construct a hypothetical revenue model for KYV-101 in treating Systemic Sclerosis, we will need to make some assumptions and provide placeholder estimates for various parameters. While we lack specific information about KYV-101's effectiveness in Systemic Sclerosis, we will base the model on general pharmaceutical industry standards and considerations for similar drugs treating complex autoimmune diseases.
Hypothetical Revenue Build for KYV-101 in Systemic Sclerosis
- Target Population Size (U.S. and Europe): Let's denote "P" as the estimated prevalence of Systemic Sclerosis in the combined markets. For instance, P = 300,000 patients.
- Penetration Rate / Access to Treatment: This is the percentage of the total target population we expect to be treated with KYV-101.
- Year 1 Post-Approval: 0.5% penetration
- Year 2 Post-Approval: 1% penetration
- Year 3 Post-Approval: 2% penetration
- Insurance Coverage: Denoting "I" as the proportion of patients with adequate insurance coverage. Assume I = 80%.
- Duration of Therapy: Assumed to be ongoing or chronic, denoted as "T." As Systemic Sclerosis is a chronic disease, T = 12 months.
These revenue projections are very simplistic and exclude many factors that can affect revenue, such as competitive landscape, market dynamics, regulatory milestones, post-marketing surveillance, and potential label expansions. Also, they do not consider the costs associated with the development, marketing, and distribution of the therapy.
Myasthenia gravis
To construct a hypothetical revenue model for KYV-101 in treating Myasthenia Gravis (MG), let's assume certain market conditions and standard pricing approaches for novel therapies. The following are estimations and placeholders:
- Prevalence (Target Population Size): For this hypothetical scenario, let's assume that the target population for MG in the markets where the company operates is "P" individuals. For example, P = 60,000 patients.
- Treatment Penetration Rate: This is based on the percentage of the total addressable market (TAM) that the drug is expected to capture.
- Year 1 Post-Approval Penetration: We'll assume a 1% penetration rate.
- Year 2: 2% penetration rate.
- Year 3: 3% penetration rate.
- Insurance Coverage Rate: The percentage of patients with insurance coverage expected to reimburse the cost of treatment. Let's denote this as "I" with a value of 80%.
- Duration of Therapy: This represents how long patients are expected to be on the therapy, which would be influenced by the nature of MG as a chronic condition and the treatment protocol of KYV-101. For simplification, we will assume the average duration is one year (T = 12 months).
This hypothetical revenue model is simplified and meant to illustrate potential revenue based on these assumptions. It does not include other potential costs such as production, marketing, post-market studies, potential price increases, or payer and reimbursement landscape shifts. Actual revenues would also be influenced by the outcomes of the clinical trials, product launch, competition, and the effectiveness of the commercial strategy employed.
We can estimate the probability of success for KYV-101 in Myasthenia Gravis (MG) at different clinical development stages:
Multiple sclerosis
Creating a hypothetical revenue build for KYV-101 in treating Multiple Sclerosis (MS) requires assumptions on various factors such as market penetration, pricing, discounts, insurance coverage, and treatment duration. The following are placeholder estimates for constructing this revenue model:
- Target Population Size (U.S. and EU): Let's assume "P" represents the combined prevalence of MS in the U.S. and EU markets. For this example, P = 1,000,000 patients.
- Treatment Penetration Rate: This is the percentage of the total target population expected to be treated with KYV-101.
- Year 1 Post-Approval Penetration: 0.1% penetration rate = 1,000 patients
- Year 2: 0.2% penetration rate = 2,000 patients
- Year 3: 0.3% penetration rate = 3,000 patients
- Insurance Coverage Rate: Denoted as "I," the proportion of patients with insurance coverage that will reimburse the treatment. Assume I = 85%.
- Duration of Therapy: Assume "T" as the average duration of therapy use, which for chronic conditions like MS could be ongoing. In this example, T = 12 months (1 year).
This hypothetical revenue projection is a simplified model and would need to be refined with additional data such as market dynamics, competition, cost of goods, and clinical trial outcomes that could impact approval and uptake rates. In addition, changes in regulations, insurance coverage patterns, and healthcare system dynamics can also influence these projections.
KVY-202
Therapeutic rationale
The therapeutic rationale for using KYV-201, an allogeneic CD19 CAR T-cell product candidate, in the treatment of undisclosed autoimmune diseases is based on the hypothesis that transient suppression of B cells could reset the immune system and provide long-term durable responses. In autoimmune diseases, aberrant B cells often play a crucial role by producing autoantibodies that attack the body's own tissues. CD19 is a protein expressed on the surface of B cells, which makes it an ideal target for CAR T-cell therapies aimed at depleting B cells.
While autologous CAR T-cell therapies have been the focus for cancer treatment due to their long persistence and adaptability to patients, the transient nature of allogeneic CAR T-cells could be an advantage in autoimmune diseases as a shorter-term effect might suffice. If successful, KYV-201 could offer the benefit of being an "off-the-shelf" therapy, eliminating the need for personalized manufacturing and potentially providing a quicker and more cost-effective treatment option.
Addressing immunological challenges is paramount in allogeneic T-cell therapy. The Intellia gene editing technology is employed to minimize the risk of graft-versus-host disease (GvHD) by eliminating the expression of the T-cell receptor (TCR) on donor cells. This effectively prevents the infused T cells from attacking the patient's healthy cells.
Moreover, to avoid rapid elimination by the patient's immune system, the Intellia strategy partially knocks out HLA alleles on the donor T cells. This nuanced approach is intended to make the cells less recognizable to the patient's natural killer (NK) cells, which could destroy cells lacking HLA entirely, while still allowing some degree of recognition by T cells to prevent immediate rejection.
Preclinical studies have shown that the gene edits intended for KYV-201 do not impair the CAR T cells' capacity for cytotoxicity and cytokine production, which are crucial for their anti-B-cell activity. Therefore, based on this data, KYV-201 could potentially deliver the therapeutic benefit of reducing or eliminating pathological B cells in autoimmune diseases without the long-term complications associated with persistent B-cell suppression.
Ultimately, the success of allogeneic CD19 CAR T-cell therapy in autoimmune diseases will depend on clinical trial results demonstrating efficacy and safety, including the management of potential adverse events such as cytokine release syndrome and neurotoxicity, which are observed in CAR T-cell therapies for oncology. If successful, KYV-201 could provide a novel therapeutic avenue for patients suffering from various B-cell mediated autoimmune diseases.
The science underlying the use of allogeneic CD19 CAR T-cell therapy in autoimmune diseases is an emerging area and represents an extension of the established applications of CAR T-cell therapies in oncology, particularly in B-cell malignancies such as certain leukemias and lymphomas. While the principles of CAR T-cell therapy are well established in cancer treatment, its application in autoimmune diseases is still in the experimental and exploratory stage and there are several key uncertainties and points of scientific debate:
- Durability and Depth of B-cell Suppression: The notion that transient B-cell depletion could reset the immune system in autoimmune diseases is intriguing but not yet proven. It is based on the hypothesis that a temporary reduction of autoreactive B cells will be sufficient to disrupt pathogenic processes. However, the evidence for this in clinical settings is limited and requires further investigation.
- Immunological Rejection: Allogeneic CAR T-cell therapy must address the issue of graft rejection by the host immune system. Strategies to prevent this, such as gene editing to reduce the expression of TCR to avoid GvHD and the partial knockout of HLA to escape NK cell killing, are innovative but still subject to ongoing research and clinical validation.
- Safety Profile: The safety profile of allogeneic CAR T-cells in autoimmune diseases has yet to be established. Issues like cytokine release syndrome (CRS) and neurotoxicity that are encountered in oncological CAR T-cell therapies may also present themselves in the treatment of autoimmune diseases.
- Off-the-Shelf Availability: The advantage of having an "off-the-shelf" product is significant in terms of accessibility and cost. However, ensuring that these allogeneic cells are universally compatible and maintain their efficacy across a diverse patient population is an ongoing challenge.
- Gene Editing: The use of gene editing in the development of KYV-201 is sophisticated and represents a cutting-edge technology in this field. While Intellia's CRISPR-based gene editing is a promising tool, the long-term consequences of gene editing in human patients and its safety and efficacy are still areas of investigation and debate.
Overall, the level of evidence supporting the processes described for allogeneic CD19 CAR T-cell therapy in the context of autoimmune diseases is still in the preclinical or early clinical stages. While initial preclinical studies appear promising, these findings need to be replicated and validated in clinical trials. Consequently, significant research and clinical development work remains before this therapy can be considered established in the field of autoimmune disease treatment. Ultimately, the viability of this strategy will be determined by clinical trial outcomes and the ability of this therapy to demonstrate clear benefits over existing treatments.
Platform and strategy
Kyverna Therapeutics' overall scientific strategy focuses on delivering innovative cell therapy product candidates for autoimmune diseases. Their approach leverages the success of Chimeric Antigen Receptor (CAR) T-cell therapies in oncology and applies it to autoimmune conditions, where pathologic B cells contribute to disease pathogenesis by producing autoantibodies that attack the body's own tissues.
Core Strategies
- Cell Therapy for Autoimmune Diseases: Kyverna is focusing on transforming the treatment paradigm by offering targeted cell therapies for autoimmune patients, particularly those who have not had success with other treatments.
- Clinical Advancement of KYV-101: KYV-101 is a CD19-targeted CAR T-cell therapy currently in clinical trials, signaling its potential in lupus nephritis, a form of autoimmune kidney disease.
- Development of KYV-201: This represents Kyverna’s effort in creating "off-the-shelf" allogeneic therapies derived from healthy donors, aiming to make treatments more accessible and avoid the complexity of autologous therapies that require patient-specific cells.
- Investigator-Initiated Trials: They encourage and support external clinical trials led by investigators and named patient programs to gather more data and increase the adoption of their therapies if approved.
- Acquisition of Differentiated Technologies: Kyverna invests in acquiring or partnering to obtain novel technologies to expand their pipeline, including T-regulatory (T-reg) cell therapies and novel CAR constructs.
- Preparing for Commercialization: The company intends to grow into an integrated biopharmaceutical company by investing in manufacturing capabilities and strategic partnerships.
Scientific Background
- CD19 CAR T-Cell Therapy in Autoimmune Diseases: CD19 CAR T-cell therapy, already established in treating B-cell malignancies, is now being explored for autoimmune diseases since both disorders involve pathologic B cells. Early clinical data shows potential for remission in diseases such as systemic lupus erythematosus (SLE).
Potential Benefits
- Broad Application: CD19 CAR T-cell therapy may be applicable to a variety of B-cell driven autoimmune diseases, suggesting a wide-reaching impact.
- Long-Term Benefits: Preliminary case studies suggest these therapies could lead to significant long-lasting clinical benefits and potentially reset the immune system.
- Improved Safety Profile: Understanding and management of CAR T-cell therapy-related side effects, such as cytokine release syndrome (CRS), have improved, potentially making these therapies safer and more tolerable.
Kyverna is also exploring T-regs and new CAR constructs to address a broader range of autoimmune diseases, such as IBD.
Potential Risks and Pitfalls
- Clinical Trial Outcomes: The success depends heavily on the outcomes of ongoing and future clinical trials to demonstrate efficacy and safety.
- CRS Management: While advancements have been made, managing the risks associated with CRS is crucial, especially as CAR T-cell therapies extend into autoimmune diseases.
- Manufacturing Challenges: Autologous CAR T-cell therapies are complex and costly to manufacture, requiring a significant amount of time from cell extraction to reinfusion. These challenges need to be overcome to ensure efficient treatment delivery.
- Allogeneic Therapy Complexities: The creation and use of allogeneic ('off-the-shelf') therapies bring their own sets of challenges, including immune rejection and potential for less individualized treatment responses.
- Competition and Innovation: Rapid advances in treatments for autoimmune diseases mean that Kyverna must continue to innovate and compete with a growing number of companies in the cell therapy space.
- Regulatory Hurdles: Gaining regulatory approval for new treatment modalities can be lengthy and unpredictable, posing financial and operational risks.
Kyverna's scientific strategy is ambitious and if successful, could translate the transformative potential of CAR T-cell therapies from oncology to autoimmune diseases. However, the approach is not without significant challenges and uncertainties that need to be navigated with careful clinical development and strategic foresight.