MBrace Therapeutics investment analysis
November 14, 2023
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Overview
MBrace Therapeutics, Inc. is a biopharmaceutical company developing treatments for cancer, primarily through antibody-drug conjugates (ADCs). These ADCs are designed to selectively destroy cancer cells while minimizing damage to healthy tissue, potentially reducing side effects compared to conventional chemotherapy.
The company's flagship product, MBRC-101, is an ADC targeting the EphA5 receptor tyrosine kinase, which is often abundant in various difficult-to-treat cancers. MBrace utilizes its SPARTA platform to streamline the production of these targeted therapies, which could reshape the treatment landscape for cancers lacking satisfactory options.
After securing $85 million in Series B funding, MBrace plans to propel its clinical programs forward, including a promising first-in-human trial for MBRC-101.
Pipeline overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial |
|---|---|---|---|---|---|---|---|---|---|---|
| MBRC-101 | Antibody-drug conjugate | EphA5 ADC | Solid tumors |
Highlights and risks
EphA5 is a potentially attractive target due to expression on many types of solid tumor and limited expression in adult healthy tissue
Ongoing Phase 1 study has potential to show early proof-of-concept
Significant strategic interest in ADCs due to strong recent clinical and commercial performance
Science supporting EphA5 as a cancer target, while promising, is more preliminary than that of some other cancer targets
If not appropriately targeted, ADCs have potential for toxicity
Oncology is a highly competitive space with many studies competing for enrollment
Solid tumor therapeutics are a challenging, risky and competitive area for drug development
Valuation
Given the early stage of the company and limited information about its programs, we did not conduct a valuation analysis.
Platform overview
MBrace Therapeutics leverages SPARTA (Selection of Phage-displayed Accessible Recombinant Targeted Antibodies) as the cornerstone of their scientific strategy for developing targeted therapies for cancer. The SPARTA methodology is designed to streamline the discovery of human monoclonal antibodies with high specificity for tumor cell surface targets, facilitating the rapid transition from discovery to clinical application.
The overall flow of the SPARTA strategy is as follows:
In Vitro Screening: A naive human antibody library is screened against predetermined tumor cell surface targets using phage and/or yeast display technologies. This high-throughput process enriches a preliminary pool of antibodies with specificity to the known tumor targets.
In Vivo Selection: Following in vitro screening, the pre-enriched antibody pool undergoes further selection in vivo to determine tumor-homing capabilities. This exploits the native tumor microenvironment, which is crucial since in vitro conditions cannot fully replicate the complexity of biological systems.
Pipeline: Once selected, the antibodies proceed through sequencing and expression (to produce the antibodies), characterization (to understand their specificities and biological activities), and in vivo testing (for therapeutic efficacy and safety).
SPARTA claims to overcome usual challenges associated with monoclonal antibody development, such as the high cost, extensive time requirements, and potential lack of translational value of traditionally discovered antibodies. By enriching a pool of potential antibodies first in a high-throughput in vitro system and then in a more physiologically relevant in vivo setting, SPARTA seeks to parallelize the process of antibody discovery while also ensuring the functional relevance of the antibodies in a natural context.
The SPARTA platform has several unique features that distinguish it from other antibody discovery platforms:
- Seamless Integration of In Vitro and In Vivo Processes
- Tailored Selection Process: In SPARTA, the in vitro and in vivo processes are not just sequentially added but are intricately linked. The in vitro phase is specifically designed to enrich for antibodies that are likely to be successful in the subsequent in vivo phase. This tailored approach is different from just performing a standard in vitro screen followed by an arbitrary in vivo test.
- Optimized for Translation: The methodology is optimized to ensure that the antibodies selected in vitro are not only specific to the target but also possess properties that make them likely to be effective in a living organism, which is a critical aspect often missed in traditional methods.
- Technological Innovations
- Advanced Screening Techniques: SPARTA utilizes sophisticated phage and yeast display technologies in the in vitro phase, which are high-throughput and capable of isolating highly specific antibodies. This level of specificity is essential for the success of the in vivo phase.
- Helper Plasmid Technology: The use of helper plasmid technology in SPARTA for multivalent antibody phage display is a significant advancement. It addresses common issues like low antibody display levels and nonspecific binding, which can hinder the effectiveness of in vivo selection.
- Contextual Selection in Tumor Microenvironment
- Real-World Tumor Targeting: SPARTA's in vivo selection is conducted in a context that mimics the actual tumor microenvironment. This step is crucial for identifying antibodies that not only bind to their targets but also navigate the complex environment of a tumor, including penetrating the tumor mass and evading immune responses.
- Direct Relevance to Human Cancer Therapy: By testing in a tumor-bearing animal model, SPARTA directly evaluates how well these antibodies perform in a scenario that closely resembles human cancer, which is a significant step toward clinical application.
- Broad Applicability and Efficiency
- Versatility for Various Targets: The method has been validated for different tumor targets, showing its broad applicability.
- Rapid Translation Potential: The integrated approach, combined with the use of human antibody libraries, speeds up the process of translating laboratory findings into clinical applications.
MBrace Therapeutics' utilizes these antibodies to develop Antibody-Drug Conjugates (ADCs), which are a class of targeted cancer therapies that combine the specificity of antibodies with the cell-killing ability of cytotoxic drugs. This is evidenced by their validation studies on tumor cell surface targets like EphA5 and GRP78, where SPARTA-derived antibodies have been successfully used to create effective ADCs.
This approach is reminiscent of other existing antibody discovery technologies such as phage display and yeast display platforms, which have been employed by numerous companies for decades. Notably, phage display was awarded the Nobel Prize in Chemistry in 2018 due to its profound impact on drug discovery.
However, like any scientific method, SPARTA has potential risks and pitfalls:
Technical Complexity: The technology relies on multiple complex processes, each of which must be finely tuned to ensure success.
Specificity: While SPARTA seeks to increase the specificity of antibodies for tumor cells, there's always a risk of off-target effects or insufficient selectivity.
Efficacy in Humans: Success in preclinical models doesn't always translate to efficacy in human patients due to differences between model systems and the complexity of human biology.
Regulatory and Developmental Challenges: Even after identification and in vivo validation, antibodies must undergo rigorous testing in clinical trials, which can be time-consuming and costly.
Background on ADCs
Antibody-drug conjugates (ADCs) are a class of targeted cancer therapies that combine a monoclonal antibody specific to antigens present on the surface of cancer cells with a cytotoxic (cell-killing) agent. The antibody serves as a delivery vehicle, bringing the toxic agent directly to the cancer cells in order to minimize systemic exposure and, thus, reducing toxicity to healthy tissues.
An ADC consists of three components: the antibody, the cytotoxic drug, and a linker that connects the drug to the antibody. The antibody portion of an ADC binds to specific antigens expressed by the cancer cells. Once bound, the ADC can be internalized by the cell, whereupon the linker is cleaved, and the cytotoxic drug is released to exert its lethal effect, typically by interfering with cell division or DNA replication.
Advantages of ADCs:
- Targeted Delivery: The ‘guided missile’ approach improves the therapeutic index by concentrating the drug in the vicinity or inside the tumor cells while sparing healthy cells.
- Reduced Side Effects: As the cytotoxic agent is not released systemically at high levels, ADCs can often lead to fewer side effects than traditional chemotherapy.
- Potential for Overcoming Drug Resistance: ADCs can potentially overcome resistance to other forms of chemotherapy by delivering cytotoxic agents that are not subject to the same resistance mechanisms.
- Combination Therapy: ADCs can be used in tandem with other cancer treatments, potentially leading to synergistic effects.
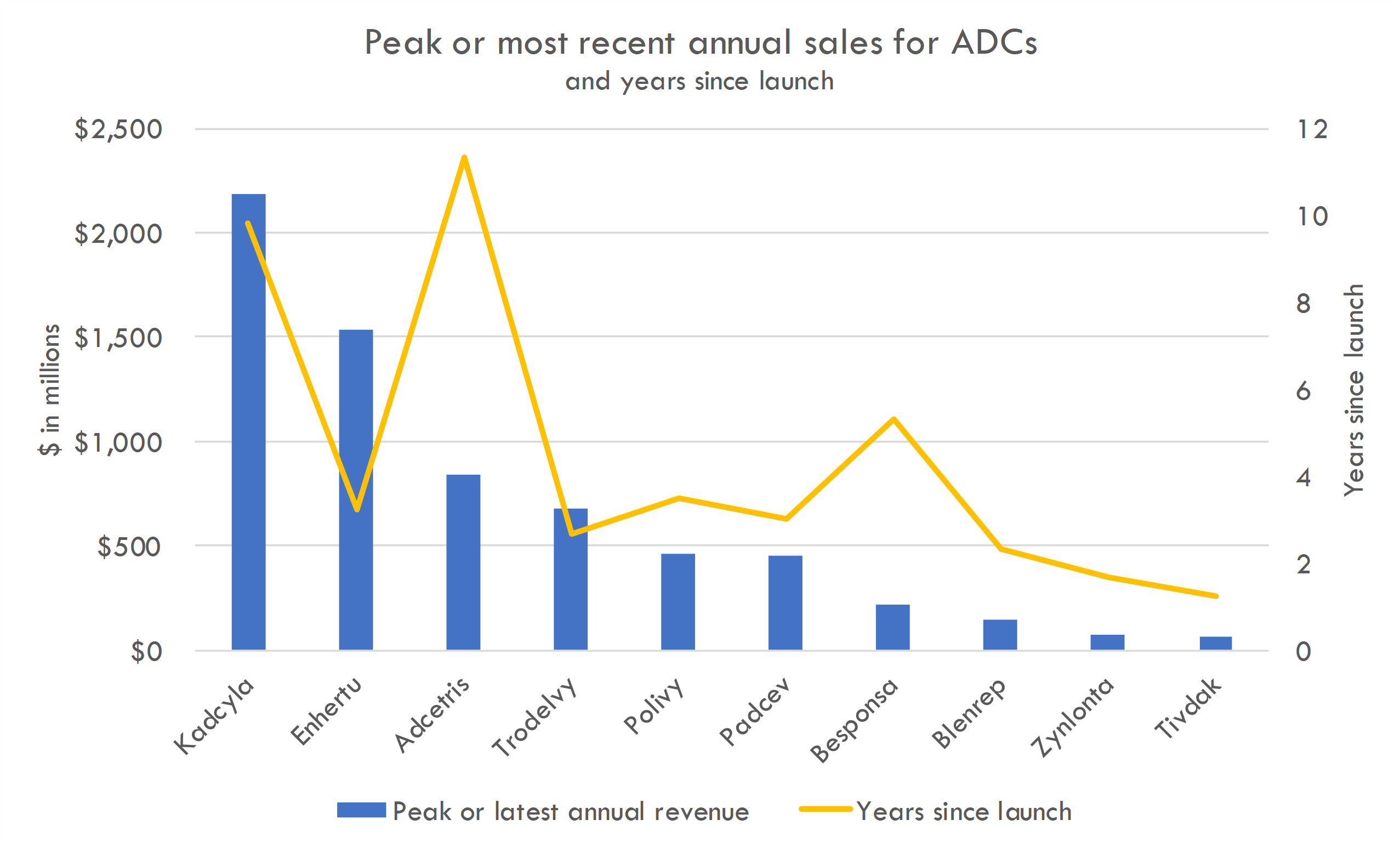
ADCs have generated strong clinical data in recent years in solid tumors, with 15 current FDA approved ADCs for cancer treatment. Enhertu, an ADC that combines trastuzumab, a monoclonal antibody targeting HER2, with a cytotoxic agent, has shown significant efficacy in treating HER2-positive breast cancers, particularly in patients who have previously undergone multiple lines of treatment.
Combination therapies have also shown promise. KEYTRUDA, an anti-PD-1 therapy, and Padcev, an ADC targeting Nectin-4, a protein found in high amounts in urothelial cancers, showed a 53% reduction in the risk of death compared to chemotherapy in patients with untreated locally advanced or metastatic urothelial cancer in the Phase 3 KEYNOTE-A39 trial. Datopotamab Deruxtecan plus Imfinzi (Durvalumab) showed promising efficacy in initial results from the TROPION-Lung04 Phase Ib trial in advanced non-small cell lung cancer (NSCLC).
Recently, many large pharma companies have acquired or partnered with ADC developers. Big pharma companies like Bristol Myers Squibb (BMS), GSK, and Merck have been aggressively pursuing deals in the ADC space. Notably, Merck made a significant $4.5 billion upfront deal with Daiichi Sankyo, potentially totaling $22 billion. The interest in ADCs has led to high valuations and competitive bidding for promising ADC technologies and candidates. Some companies, like Lilly, are choosing to invest in earlier-stage, preclinical ADC opportunities, accepting higher risks for potentially greater long-term value. Others, like Regeneron, prefer to focus on internal ADC development efforts.
Despite their promise, ADCs face significant challenges and limitations.
Challenges and Limitations for ADCs Targeting Solid Tumors:
- Tumor Accessibility: Solid tumors often have poor vascularization and a dense extracellular matrix, making it difficult for ADCs to penetrate and distribute evenly within the tumor.
- Heterogeneity: Solid tumors can be highly heterogeneous, with differing antigen expression levels, which might result in uneven targeting and treatment.
- Antigen Selection: Identifying suitable antigens that are highly expressed on tumor cells but minimally on normal cells is critical to the success of an ADC.
- Off-target Toxicity: Despite targeted delivery, some ADCs can still affect healthy tissues if the antigen is also present (albeit at lower levels) on non-cancerous cells.
- Linker Stability: The chemical linker between the antibody and the cytotoxic drug must be stable enough to remain intact in the bloodstream but also be cleavable once inside the cancer cell.
- Resistance: Mechanisms of resistance to ADCs can develop, including changes in antigen expression, drug efflux, and alterations in cell death pathways.
MBrace Therapeutics' SPARTA platform seems designed to address several of the key challenges associated with developing ADCs for the treatment of solid tumors. Here's how their approach potentially addresses these challenges:
Tumor Accessibility and Antigen Selection: SPARTA begins with in vitro screening against known tumor targets. This high-throughput process aims to select antibodies that are highly specific to antigens overexpressed on the surface of tumor cells, ensuring that once an ADC is formed, the targeting component of the conjugate will direct the cytotoxic payload precisely to the cancer cells. By using both in vitro and in vivo selection methods, SPARTA ensures that selected antibodies are more likely to bind effectively and be internalized by tumor cells, enhancing their accessibility to solid tumors.
Heterogeneity: The in vivo selection step of the SPARTA methodology considers the microenvironment of the tumor, which is critical given the heterogeneity of solid tumors. This approach attempts to select antibodies that not only target specific antigens but also are effective in the complex ecosystem of a tumor, potentially leading to more uniform tumor penetration and efficacy across varying tumor cell populations.
Off-target Toxicity: By using an approach that is both high-throughput and highly selective, MBrace Therapeutics aims to minimize off-target toxicity. Their platform is designed to identify antibodies with favorable tumor-targeting attributes, which should increase the therapeutic index of their ADCs (the ratio of effective therapeutic dose to the dose at which the drug is toxic).
Linker Stability: While MBrace has not detailed how they specifically address linker chemistry, the choice of linker and cytotoxic drug is a critical component of ADC development. Ensuring the stability and cleavability of the linker is often a significant part of preclinical studies, and MBrace"s methodology likely involves optimizing these components alongside antibody selection.
Resistance: The SPARTA methodology allows for the discovery of a wide array of antibodies against a single antigen or multiple different antigens. This wealth could provide options for combination treatments or switching ADCs in case resistance develops, a common issue in targeted cancer therapies.
It's important to note that while the SPARTA platform may address various challenges associated with the development of ADCs, MBrace Therapeutics would still need to conduct extensive preclinical and clinical testing to validate the effectiveness and safety of their ADC candidates. The transition from the identification of promising antibodies to the development of safe and efficacious ADC therapeutics can be long and complex, with regulatory hurdles and the necessity for large-scale manufacturing solutions. However, innovations like MBrace Therapeutics' SPARTA could potentially streamline aspects of this process and bring much-needed therapies to the clinic more rapidly.
Technical risks
While the SPARTA technology from MBrace Therapeutics has potential, there are several technical risks and limitations that could be encountered in the development of antibody-drug conjugates (ADCs):
Complexity of Antibody Selection: The robustness of the SPARTA method relies on identifying antibodies that both bind specifically to tumor cells and possess tumor-homing capabilities. However, the complexity of this tandem process of in vitro and in vivo selection may lead to unforeseen technical challenges. For example, phage display and in vivo biopanning require careful optimization to select for antibodies with the desired properties.
Scalability and Reproducibility: Translating the antibody discovery process from a smaller-scale, controlled laboratory setting to scalable production that is consistent and reliable can be challenging. Achieving a reproducible process that maintains antibody specificity and efficacy is necessary for clinical and commercial success.
Linker Chemistry and Drug Conjugation: Although SPARTA focuses on the selection of antibodies, the development of ADCs also depends critically on the linker and cytotoxic drug. Creating a stable, cleavable linker and conjugating it without affecting the antibody binding properties requires sophisticated chemistry and may present obstacles.
Preclinical to Clinical Translation: Antibodies and ADCs, which show promise in preclinical models, do not always translate successfully in clinical trials. The tumor microenvironment in patients can differ significantly from that in preclinical models, which may affect the ADC's efficacy and safety profile.
Off-Target Toxicity and Immunogenicity: Even with highly specific antibody selection, there's a possibility of cross-reactivity with other tissues or the induction of an immune response against the ADC, which can lead to off-target toxicity and reduced therapeutic window.
Intellectual Property: As the ADC field is competitive with many players, intellectual property rights could be a limiting factor. MBrace must navigate existing patents and ensure their technology is sufficiently differentiated to avoid infringement and to secure their own proprietary space.
Competition: There are a number of well-established companies and start-ups in the ADC arena employing different technologies for antibody discovery and ADC optimization. Some use alternative antibody formats or novel cytotoxic payloads that might offer advantages over conventional approaches, potentially outpacing SPARTA if it cannot demonstrate superior selection or efficacy.
Financial Consideration: The cost of research and development for ADCs is high. MBrace Therapeutics may face financial challenges as it scales up its operations and progresses through costly clinical trials, especially if competing technologies are more cost-effective.
Regulatory Hurdles: Even with a powerful discovery platform, obtaining regulatory approval is a complex process. Any new technology or modality will be closely scrutinized by health authorities to ensure safety and efficacy, which carries both time and financial risks.
In summary, MBrace Therapeutics' approach using SPARTA presents an innovative way to select antibodies for ADC development that may overcome some of the limitations in the field. However, they must navigate technical and competitive hurdles in the rapidly evolving landscape of ADC technologies to establish their approach as a frontrunner. Being aware of these potential risks and limitations and having strategies in place to address them will be crucial as they progress toward clinical development.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
MBRC-101
Scientific thesis
The therapeutic rationale for an EphA5 receptor tyrosine kinase antibody-drug conjugate (ADC) in solid tumors in the preclinical stage as developed by MBrace Therapeutics involves exploiting the unique expression patterns of EphA5 in various cancers and the precise delivery mechanism that ADCs offer.
EphA5 is a member of the erythropoietin-producing hepatocellular (Eph) receptor tyrosine kinase family, which is implicated in mediating developmental events, particularly in the nervous system.
Beyond their roles in development, Eph receptors and their ephrin ligands have been found to contribute to the regulation of cancer-related processes including angiogenesis, metastasis, and tumor cell survival. While research on EphA5 in cancer is not as extensive as for some other members of the Eph receptor family (like EphA2 or EphB4), there is growing evidence of its involvement in various cancers. Studies have linked EphA5 to the progression of certain solid tumors, including breast, lung, colorectal, gastric, and pancreatic cancers.
EphA5 expression in cancer has been observed in multiple solid tumor types. However, the frequency and intensity of its expression can vary widely depending on the tumor type and individual patient characteristics.
In adults, its expression in normal tissues appears to be limited or at least significantly lower compared to its upregulation in certain cancer types.
The differential expression in cancerous versus normal tissues makes it an attractive target for ADCs, as targeted therapies aim to minimize off-target effects on normal cells.
EphA5 presents as a promising target for ADCs in solid tumors based on its role in cancer progression and expression patterns. However, the current understanding is still evolving, and more clinical data are needed to fully establish EphA5's role in cancer and its viability as a therapeutic target.
Clinical trial overview
MBRC-101 is being studied in a Phase 1/1b, open-label, multi-center study for advanced refractory solid tumors in a first-in-human trial. The two-phase study aims to establish safety, pharmacokinetics, and preliminary efficacy:
- Phase 1 (Dose Escalation): Involves ~30 patients to find the optimal biologically relevant doses (OBRDs) and the maximum tolerated dose (MTD). The phase does not require EphA5 expression for enrollment but will evaluate it retrospectively.
- Phase 1b (Dose Expansion): Focuses on ~60 further patients spread across three cohorts to examine safety and preliminary clinical activity at OBRDs. Efficacy is gauged through various response metrics (ORR, PFS, RR, OS, CR, PR) per RECIST v1.1 criteria via imaging techniques (PET-CT, CT, MRI).
Safety is continuously monitored by a Safety Review Committee (SRC), and the study also characterizes single and multiple-dose pharmacokinetics as well as the incidence and persistence of anti-MBRC-101 antibodies.
Both phases aim to characterize the pharmacokinetic (PK) profiles of single and multiple doses and to evaluate the incidence and persistence of anti-MBRC-101 antibodies (Abs).
Phase 1 will involve dose escalation with approximately 30 patients to determine MTD, while expression of EphA5 is not a criterion for enrollment but will be assessed retrospectively. Phase 1b includes dose expansion to further evaluate safety and preliminary efficacy, with three expansion cohorts estimated at around 60 total patients. Safety will be monitored by a Safety Review Committee (SRC) throughout both phases.
Efficacy will be measured by overall response rate (ORR), progression-free survival (PFS), response rate (RR), overall survival (OS), and complete and partial responses (CR and PR), assessed per RECIST v1.1 criteria using PET-CT, CT, and MRI scans. The expected study duration is from November 2023 to October 2025, with an estimated enrollment of 90 patients.
Critiques of Study Design:
- Open-Label Design: The lack of a control arm or blinding could introduce bias in reporting and assessment of outcomes, although this is not uncommon in early-phase oncology trials.
- Sequential Assignment: The design assumes that earlier cohorts will not influence later ones, which might not hold if, for instance, adverse events lead to changes in the study protocol.
- Retrospective EphA5 Expression Assessment: Not selecting patients upfront based on EphA5 might dilute the efficacy signal if the agent is particularly effective in EphA5-positive tumors.
Operational and Technical Challenges
- Patient Recruitment: Recruiting patients with advanced, refractory solid tumors can be challenging, as they may be enrolled in or considering other studies or treatments.
- Pharmacokinetics Characterization: The study must manage extensive and potentially complex PK sampling and analysis schedules.
- Imaging Requirements: Standardizing and ensuring the quality of RECIST assessments with PET-CT, CT, and MRI might present logistical challenges, particularly across multiple centers.
- Anti-Drug Antibody Monitoring: Development and validation of assays to monitor anti-MBRC-101 antibodies will need to be rigorously managed, as false positives or negatives could impact safety and efficacy assessments.
- Adverse Event Management: Given the likely occurrence of treatment-emergent adverse events and dose-limiting toxicities particularly in a population with advanced disease, managing and treating these while maintaining the integrity of study data will be challenging.
- Continual assessment of anti-drug antibodies could require rigorous and sensitive assay development to ensure accurate detection of immunogenicity.
- Managing multiple cohorts with separate dosing regimens may pose logistical complexities in ensuring proper dose administration, capturing accurate data, and maintaining the safety of participants.
- Rigorous training and standardization of the imaging analysis as per RECIST v1.1 criteria across different centers are crucial to minimize inter-observer variability.
- Managing adverse events and determining dose-limiting toxicities with an entirely new agent will require careful oversight by the SRC, which may be challenging in a multi-center trial.
Overall, this study design is typical of early-phase clinical trials in oncology aimed at evaluating new treatments where the primary focus is on establishing the dosing and safety profile. The study design of MBRC-101 appears to be rigorous in its approach to determine the MTD, evaluate safety, pharmacokinetics, and preliminary efficacy of an anti-EphA5 MMAE antibody-drug conjugate in a particularly vulnerable patient population.
Potential for Proof-of-Concept:
The study design and inclusion/exclusion criteria for MBRC-101 seem well-constructed to provide proof-of-concept for its use in solid tumors. The selection of a population with advanced metastatic solid tumors refractory to standard treatment is appropriate for a first-in-human trial, as these patients have an unmet need for new therapeutic options.
The primary and secondary endpoints are adequately chosen:
- Primary endpoints such as the occurrence of treatment-emergent adverse events (TEAEs) and dose-limiting toxicities (DLTs) are standard for Phase 1 studies and essential for establishing the safety profile of MBRC-101.
- Secondary endpoints, including objective response rate (ORR), duration of response (DOR), and progression-free survival (PFS), are appropriate for evaluating preliminary efficacy and align well with the RECIST v1.1 criteria for solid tumors.
Appropriateness of Inclusion/Exclusion Criteria:
The inclusion criteria properly define the patient population and ensure that participants can consent and comply with study procedures. The criteria related to reproductive status and contraception are standard for oncology trials to prevent potential drug-induced harm to fetuses or infants. The requirement for measurable disease in Phase 1b ensures that the response to the drug can be objectively assessed.
EphA5 expression is a key aspect of the study design, especially for Cohort C in Phase 1b, which targets this biomarker. This specificity increases the study's relevance as targeted therapies move towards personalization based on tumor characteristics.
The exclusion criteria are designed to protect patients from undue risk and to prevent confounding factors such as other drugs or conditions that might affect the study's outcome or the drug's pharmacodynamics. For example, excluding patients with active, symptomatic central nervous system (CNS) metastases is common as CNS involvement can complicate assessment of response and adverse events.
Reproducibility Challenges:
The use of EphA5 IHC H-score as an inclusion criterion for Cohort C (basket cohort) in Phase 1b is a potential source of variability and could pose a challenge for reproducibility. Immunohistochemistry can be somewhat subjective, and variability can occur between different laboratories. However, since the protocol specifies analysis by a Sponsor-designated central laboratory, this may help mitigate inter-lab variability.
For Cohort C, obtaining tissue samples that are representative of the current disease state could also be challenging, particularly if archival samples do not reflect current biology or if fresh samples are difficult to obtain due to the risk or patient's clinical status.
Furthermore, criteria such as "significant risk" (related to tumor biopsies) or "patients should have the ability to read and understand the ICF" can be somewhat subjective and may depend on the judgment of individual investigators, which can affect the study"s homogeneity and reproducibility.
In summary, while the eligibility criteria are generally appropriate and designed to ensure patient safety and the collection of meaningful data, potential challenges include variability in immunohistochemical assessment and subjective assessments within the criteria. As long as these criteria are applied consistently across all study sites, they should not present significant barriers to reproducibility.
Analyze investments with AI
AI puts the power of a team of analysts at your fingertips, 24/7.
Generate high-quality investment analyses in minutes, not days. From valuation analysis, to deal screening, to startup idea generation, AI can save you time and make you smarter.
Market overview
Solid tumors represent a large market opportunity in oncology due to the high incidence and prevalence of these cancers globally. Solid tumors include a wide range of cancer types like breast, lung, colorectal, prostate, and many others. These are distinguished from hematologic malignancies (cancers originating in the blood-forming tissues).
Market Opportunity and Unmet Medical Need:
The market opportunity in solid tumors is significant because these cancers account for the majority of new cancer cases and cancer-related deaths worldwide. There remains a substantial unmet medical need despite the availability of multiple treatment modalities, including surgery, radiation therapy, chemotherapy, targeted therapy, and immunotherapy. Resistance to current therapies, relapse, and metastasis are common challenges in the treatment of solid tumors.
Standard of Care:
The standard of care for solid tumors varies widely by the type and stage of cancer. Typically, early-stage solid tumors may be treated with surgery and potentially adjuvant therapies, whereas later stages may require a combination of treatments. In some cases, targeted therapies or immunotherapies that exploit specific molecular characteristics of a tumor are first-line treatments. Drugs like pembrolizumab (Keytruda), nivolumab (Opdivo), and various tyrosine kinase inhibitors are among the transformative treatments for certain solid tumors.
Examples of Successes in Solid Tumors:
Several targeted therapies and immunotherapies have achieved commercial success by improving outcomes for patients with solid tumors. These successes can help inform the market opportunity for preclinical drugs:
Pembrolizumab (Keytruda) - This immune checkpoint inhibitor has shown effectiveness in various solid tumors, including non-small cell lung cancer (NSCLC) and melanoma, and has achieved blockbuster status.
Trastuzumab (Herceptin) - A monoclonal antibody for HER2-positive breast cancer, Herceptin has long been a successful targeted therapy since its approval.
Imatinib (Gleevec) - A tyrosine kinase inhibitor used in chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs), Gleevec revolutionized treatment for these indications.
Atezolizumab (Tecentriq): An anti-PD-L1 antibody initially approved for urothelial carcinoma and NSCLC, and has expanded to include indications such as triple-negative breast cancer (TNBC) in combination with chemotherapy.
Olaparib (Lynparza): A PARP inhibitor for patients with BRCA mutations and has been approved for use in ovarian, breast, pancreatic, and prostate cancers.
Trastuzumab deruxtecan (Enhertu): An antibody-drug conjugate (ADC) targeting HER2-positive breast cancer and gastric cancer, which has shown efficacy in patients who have progressed after other treatments.
Larotrectinib (Vitrakvi) and Entrectinib (Rozlytrek): These are TRK inhibitors approved for the treatment of solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, regardless of the original site of the tumor (a tissue-agnostic approval).
Alpelisib (Piqray): Approved for use in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer.
Despite recent advances in solid tumor therapy, there is still significant unmet need. Resistance to current therapies, especially in advanced stages, is a significant challenge. There is a continuous need for novel therapeutics that can overcome or prevent resistance mechanisms. Patients with advanced or metastatic cancer often have limited treatment options, highlighting the need for innovative therapies in these stages.
Opportunity for MBRC-101
MBRC-101 is an antibody-drug conjugate (ADC) targeting the EphA5 receptor tyrosine kinase. EphA5 is implicated in several cancers, including breast, non-small cell lung (NSCLC), colorectal, gastric, and pancreatic cancers. Given that it is a targeted therapy, MBRC-101 has the potential to fit into the current standard of care as either a first-line or subsequent treatment, particularly for patients who express the EphA5 receptor. If successful in clinical trials, it could offer an additional or alternative therapy for those who have relapsed or are refractory to current treatments, or it may complement existing therapies such as chemotherapy, radiation therapy, or other targeted therapies.
Without seeing clinical data on the program or knowing which specific solid tumor indications it is being developed in, it is difficult to say where the product may fit within the treatment landscape. Solid tumor therapeutics is one of the most active and competitive areas of drug development, and successful programs must differentiate themselves from competing agents across a variety of therapeutic strategies.