OrsoBio investment analysis
November 6, 2023
This is not investment advice. We used AI and automated software tools for most of this research. A human formatted the charts based on data / analysis from the software, prompted the AI to do some editing, and did some light manual editing. We did some fact checking but cannot guarantee the accuracy of everything in the article. We do not have a position in or a relationship with the company.
Overview
OrsoBio, Inc. is a private clinical-stage biopharmaceutical company developing innovative treatments for obesity and related metabolic disorders.
The company focuses on small molecules to impact fundamental aspects of energy metabolism, offering a potentially complementary and convenient alternative to existing treatments, such as injectable GLP-1 receptor agonists.
The company raised a $60M Series A in November 2023 led by Longitude and Enavate Sciences, with participation from new investor Eli Lilly and existing investors Samsara BioCapital and NuevaBio. This brings the total raised to $97M.
The funding will be used to complete a Phase 2a of its LXR inverse agonist in SHTG/NASH, a Phase 2a of its ACC2 inhibitor for diabetes, a Phase 1b of its mitochondrial protonophore for obesity, and IND-enabling activities for other programs.
OrsoBio Pipeline Overview
| Product name | Modality | Target | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | FDA submission | Commercial | Description |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TLC-3595 | Small molecule | ACC2 inhibitor | Type 2 diabetes | Selective Acetyl-CoA Carboxylase 2 (ACC2) inhibition to improve insulin sensitivity in patients with type 2 diabetes | |||||||
| TLC-2716 | Small molecule | LXR inverse agonist | Severe dyslipidemias | Liver X Receptor (LXR) inhibition to improve plasma triglycerides and cholesterol in patients with severe dyslipidemias | |||||||
| TLC-6740 | Small molecule | Mitochondrial protonophore | Lipodystrophies | Mitochondrial protonophores to increase energy expenditure and improve metabolic and cardiovascular health in patients with lipodystrophies and other metabolic disorders | |||||||
| TLC-1235 | Small molecule | Mitochondrial protonophore | Lipodystrophies | Mitochondrial protonophores to increase energy expenditure and improve metabolic and cardiovascular health in patients with lipodystrophies and other metabolic disorders | |||||||
| ACMSD inhibitor | Small molecule | ACMSD inhibitor | Organ failure | ACMSD inhibition to augment NAD+ biosynthesis and improve mitochondrial function in patients with liver and/or kidney dysfunction |
Highlights and risks
Targeting large markets including diabetes, obesity, NASH and cardiovascular disease.
Several clinical-stage programs expected to complete proof of concept studies with Series A funding
Protonophore mechanism of action in obesity is somewhat derisked, and programs have potential ability to address issues of previous therapies in the class
Metabolic diseases and obesity are complex disorders and clinical development can be risky
Clinical trials in large indications can be costly
Targeting central metabolic pathways poses safety risks and requires careful study design and patient monitoring
Valuation
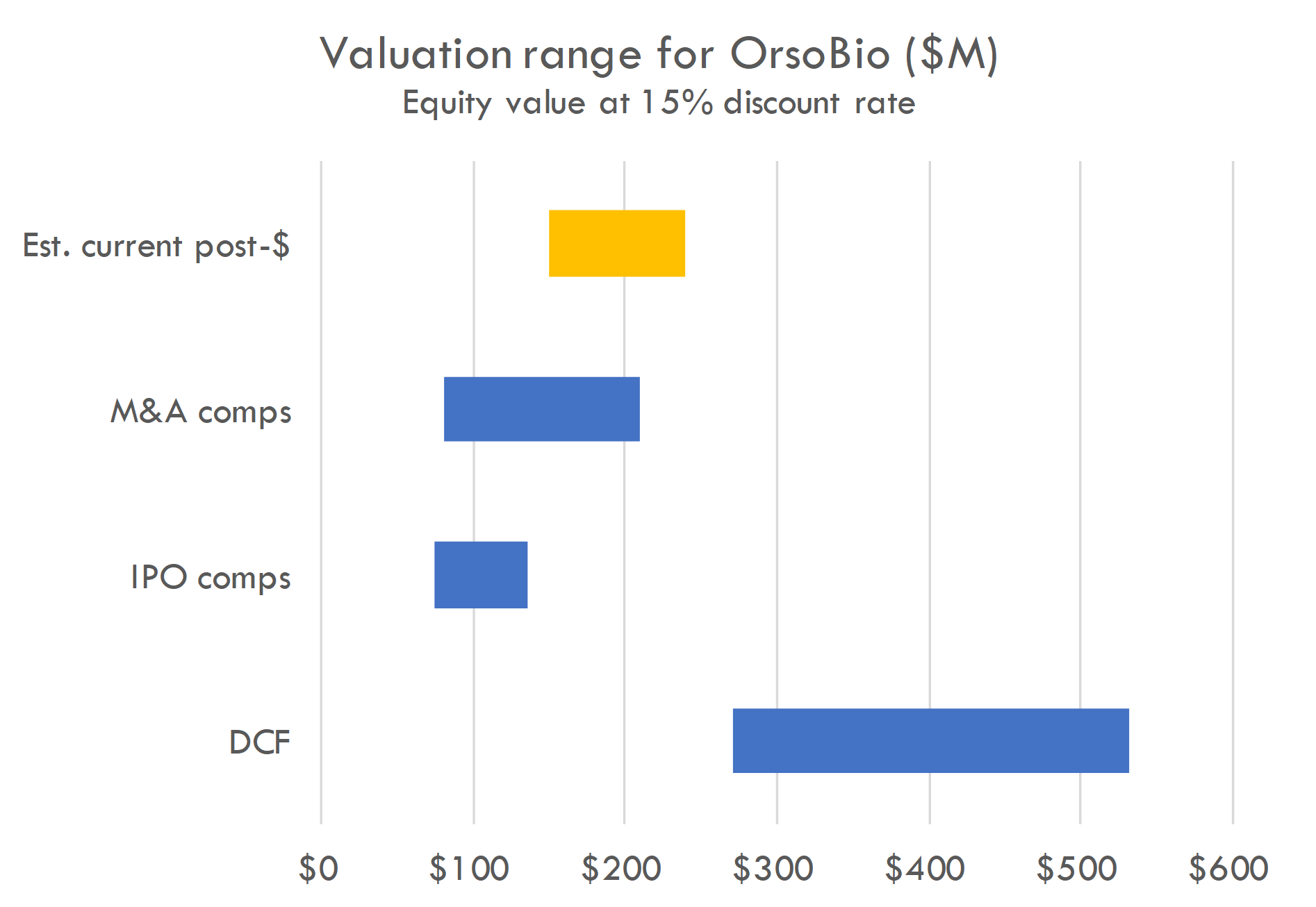
Our DCF is based on the revenue assumptions below, with industry benchmarks used for other assumptions, and a 15% discount rate. The DCF is highly sensitive to assumptions around probability success and, critically, market size. Predicting market size in large, complex indications like diabetes and obesity is challenging, and the analysis must be adjusted continually as new information becomes available.
For IPO and M&A comps, we estimate value at IPO or acquisition based on comps, estimate funding and dilution required to reach exit, and estimate probability of exit. We calculate probability-adjusted enterprise value, then discount back to the present at a 15% discount rate.
Analyze your company with our AI tools
Get a report like this one for your company or portfolio. Includes excel files with valuation analysis.
Contact us to learn more
Pipeline analysis
Mitochondrial protonophores for lipodystrophies
TLC-6740 and TLC-1235 are mitochondrial protonophores being developed to increase energy expenditure, which can lead to metabolic benefits and ultimately to weight loss, improved lipid profiles, and better insulin sensitivity.
The concept of using drugs to induce weight loss by disrupting the normal function of cell powerhouses, known as mitochondria, has been around for some time. One such drug, 2,4-dinitrophenol (DNP), makes these cellular powerhouses work less efficiently, causing the body to burn more fuel and thus increase calorie usage.
While effective for weight loss, DNP can dangerously raise body temperature, among other side effects, limiting its safe use. Mildly interfering with mitochondrial efficiency, in contrast, can help burn calories and reduce fat storage, improve the body's response to insulin, and lower unhealthy blood fat levels without the severe risks posed by drugs like DNP.
OrsoBio's compounds are designed to avoid the safety issues seen with molecules like DNP. TLC-6740 is designed to selectively target the liver, limiting its action primarily to this organ. This targeting is achieved through active hepatic uptake, meaning the drug are preferentially taken up by the liver cells, reducing its levels in the blood and, consequently, the potential to cause systemic effects like hyperthermia. TLC-6740 has high liver-to-plasma ratio facilitated by liver transporters, ensuring its action is concentrated where it's needed most. TLC-1235's controlled-release formulation likely allows for a steady, controlled release of the active compound, avoiding peaks that might lead to excessive uncoupling and thus enhancing its safety margin.
The preliminary data from Phase 1 trials suggest TLC-6740 is safe and well-tolerated, with a pharmacokinetic profile suitable for once-daily oral dosing. Their development reflects an unmet need for treatments that can have systemic metabolic effects without the toxicity associated with non-targeted mitochondrial uncoupling.
Scientific background
Protonophores are compounds that dissipate the proton gradient across the mitochondrial inner membrane. Normally, this gradient is used by the enzyme ATP synthase to generate ATP, the cell's energy currency. When protonophores disrupt this gradient (a process called "uncoupling"), mitochondria compensate by oxidizing more substrates (like fats) to restore the gradient, thus increasing metabolic rate and energy expenditure.
Increased energy expenditure leads to metabolic benefits:
- Activation of AMPK: This 'energy sensor' activates in response to increased AMP/ATP ratio due to enhanced energy demand, promoting catabolic pathways that generate ATP.
- Inhibition of DNL: AMPK activation can inhibit DNL, reducing the synthesis of new fatty acids.
- Increased Fatty Acid Oxidation: To meet the energy demand, cells oxidize more fatty acids.
- Enhanced TCA Cycle Flux: Increased substrate oxidation leads to more activity in the TCA cycle, generating energy and reducing intermediates like citrate that otherwise contribute to DNL.
Collectively, these changes can lead to weight loss (due to increased calorie burning), improved lipid profiles (from reduced DNL and increased fat oxidation), and better insulin sensitivity (as a result of altered lipid and glucose metabolism).
Mitochondrial uncoupling via synthetic protonophores like 2,4-dinitrophenol (DNP) has been historically explored as a method for inducing weight loss because it increases the basal metabolic rate. However, its use has led to significant safety concerns. Excessive systemic uncoupling can cause serious side effects, such as hyperthermia (dangerously high body temperature), which has restricted its use in clinical settings. On the other hand, mild mitochondrial uncoupling can provide metabolic benefits without these severe side effects. It can lead to increased energy consumption, reduced fat accumulation, improved insulin sensitivity, and lowered plasma lipid levels, offering a potentially safer approach to managing metabolic disorders.
Phase 1 study analysis
OrsoBio is conducting a Phase 1 study of TLC-6740. The company provided preliminary data that suggest the compound is safe and well-tolerated when administered orally once daily. In the single ascending dose (SAD) portion of the trial, mild and non-serious adverse events were reported, none of which were deemed related to the treatment. Key findings included a prolonged half-life of 17 to 46 hours and dose-dependent increases in plasma exposure.
The study design was appropriate for a Phase 1 trial, with a double-blind and placebo-controlled setup to assess safety and pharmacokinetics. The study's focus on liver targeting aims to circumvent the safety concerns associated with systemic mitochondrial uncoupling, such as hyperthermia, by ensuring active hepatic uptake of the compound. This results in lower plasma concentrations, which are projected to be much lower than those causing thermogenic side effects in preclinical studies, indicating a potentially favorable safety profile for TLC-6740. Further studies in multiple ascending dose (MAD) cohorts will be necessary to confirm these findings and elucidate the efficacy of TLC-6740 for obesity and associated metabolic disorders.
The trial enrolled a diverse group of participants across different doses ranging from 3 mg to 120 mg. The demographic data show variability in age, race, and BMI, which helps in understanding the safety and efficacy of TLC-6740 across a diverse population. TLC-6740 was tested across a range of doses from 3 mg to 120 mg. The trial included 8 subjects for each dosage group.
Reported treatment-emergent adverse events (TEAEs) were relatively low across all dosages, with the highest incidence of TEAEs (75%) at the 60 mg dose. Reported TEAEs include rhinorrhea, catheter site bruise, headache, and upper respiratory tract infection, which were all classified as mild and non-serious. There were no serious TEAEs or treatment-related TEAEs, and no subjects discontinued due to adverse events. The study completion rate was 100%, with all subjects completing dosing and follow-up.
In the context of a Phase 1 study for a drug designed to improve metabolic and cardiovascular health, the preliminary data suggest that TLC-6740 is well-tolerated across the tested dose range. The lack of serious or treatment-related adverse events is promising for the safety profile of the drug. However, as this is early-stage research, further studies, including multiple ascending dose studies, are necessary to fully understand the safety, efficacy, and optimal dosing of TLC-6740. Additionally, the effects in patients with lipodystrophies and other metabolic disorders need to be evaluated since the current study was conducted in healthy subjects.
Here is a summary of the pharmacology data from the study:
- TLC-6740 Absorption: The compound was rapidly absorbed, with peak plasma concentrations (T_max) occurring between 3 to 12 hours post-dose, which is typical for oral medications and indicates good absorption from the gastrointestinal tract.
- Half-life (t1/2): The half-life ranged from 17 to 46 hours, suggesting that once-daily dosing could maintain therapeutic levels of the drug.
- Plasma Concentration (C_max): The low maximum plasma concentration (C_max) is consistent with the drug's liver targeting properties. This means the drug is quickly taken up by the liver and does not remain in the bloodstream for an extended period, potentially reducing systemic side effects.
- Dose-Response Relationship: There were dose-dependent increases in plasma exposure, which indicates a predictable pharmacokinetic profile, important for determining appropriate dosing for subsequent trials.
- Multiple Dose Simulations: Simulations suggest that steady-state plasma and liver concentrations will be reached within 7 days of dosing. Doses of 6 mg or higher were projected to achieve therapeutic liver concentrations considered effective based on preclinical data.
- Safety Margins: The projected steady-state plasma concentrations are expected to be significantly lower than those causing adverse effects like hyperthermia in animal studies, suggesting a large safety margin for human use.
In summary, the data suggests that TLC-6740 has a favorable pharmacokinetic profile with a potential for once-daily dosing and low risk of systemic side effects due to its liver targeting. The safety and tolerability findings from this Phase 1 trial are encouraging, but efficacy and long-term safety need to be determined in subsequent clinical trials.
Protonophore market opportunity
The company is initially focused on lipodystrophies, but could potentially also develop these programs in obesity more generally. Obesity is a large market, and GLP-1 agonists have recently made notable progress in treating the disease. The market potential and pricing of the product would vary significantly depending on which indications the company pursues.
Lipodystrophies
Lipodystrophies are a group of rare, heterogeneous disorders characterized by the loss of adipose tissue, which can be either partial or total, and can be inherited or acquired. The prevalence is estimated to be very low, roughly 1 to 4 per million for generalized forms and about 1 per 100,000 for partial forms.
Patients with lipodystrophy can exhibit a range of symptoms, including a lack of subcutaneous fat, muscular hypertrophy, acanthosis nigricans, and metabolic complications such as insulin resistance, diabetes, hypertriglyceridemia, and fatty liver disease. The prognosis varies depending on the type and severity of the condition and its associated metabolic disorders
Standard care includes dietary modifications, antidiabetic medications, lipid-lowering agents, and leptin replacement therapy, particularly for those with congenital forms. However, given the rarity and complexity of the disease, many patients have unmet medical needs, including a lack of targeted treatments and difficulties in managing metabolic complications. The development of new therapies, such as those focusing on increasing energy expenditure or improving insulin sensitivity, is ongoing to address these needs. Notable drugs include:
- Metreleptin (Myalept): This is an analog of human leptin and is specifically approved for the treatment of generalized lipodystrophy. It is significant because patients with lipodystrophy have a deficiency or absence of leptin.
- Ursodeoxycholic Acid: While not specifically approved for lipodystrophy, it is sometimes used off-label for patients with fatty liver disease, including those with lipodystrophy.
- Thiazolidinediones (such as Pioglitazone): These are PPAR-gamma agonists that are used to treat insulin resistance, a common issue in patients with lipodystrophy.
- Insulin and other Diabetes Medications: Due to the high prevalence of diabetes in lipodystrophy patients, insulin and other antidiabetic drugs are commonly used to manage blood sugar levels.
- Statins and Fibrates: These are used to manage high cholesterol and triglyceride levels, which are often seen in lipodystrophy patients.
Emerging treatments for lipodystrophies are largely focused on addressing metabolic complications such as diabetes and hypertriglyceridemia. These include:
- Leptin Replacement Therapy: Metreleptin (Myalept) has been a significant advance for patients with generalized lipodystrophy, addressing leptin deficiency and improving metabolic abnormalities.
- PPAR-gamma Agonists: Drugs like pioglitazone can help manage insulin resistance and diabetes, though they are not specific to lipodystrophy.
- Glucose-lowering agents: SGLT-2 inhibitors and GLP-1 receptor agonists are being explored for their potential benefits beyond glycemic control, such as weight management and cardiovascular risk reduction.
- Lipid-lowering therapies: Fibrates, omega-3 fatty acids, and statins are used to manage hypertriglyceridemia and reduce the risk of pancreatitis.
- Novel Therapeutic Approaches: Research is ongoing into therapies that directly target the pathophysiology of lipodystrophy, such as mitochondrial protonophores like TLC-6740 and TLC-1235, which aim to increase energy expenditure and improve metabolic health.
- Gene Therapy: For hereditary forms of lipodystrophy, gene replacement or editing therapies are under investigation, though these are still in early stages of research.
It’s important to note that many of these treatments are still under clinical investigation and have not yet been approved for lipodystrophy specifically. The management of lipodystrophy remains complex, and new treatments are greatly needed.
TLC-6740, as a liver-targeted mitochondrial protonophore, represents a novel therapeutic approach for lipodystrophies. Given that lipodystrophies are characterized by abnormal fat distribution and associated metabolic complications, such as insulin resistance, dyslipidemia, and nonalcoholic fatty liver disease (NAFLD), TLC-6740 could potentially address these issues by increasing energy expenditure in hepatocytes. This could improve metabolic profiles, which is a significant unmet need in lipodystrophy care. However, its place in the standard treatment regimen would depend on the outcomes of clinical trials, especially regarding its efficacy, safety profile, and how it compares with or complements existing therapies such as leptin replacement therapy.
Revenue build
- Prevalence: Assume there are approximately 4,000-5,000 patients with lipodystrophy in the U.S.
- Treatment Line: Assuming TLC-6740 offers significant benefits, it may be used as a first or second-line treatment post-diagnosis.
- Market Penetration: If TLC-6740 proves effective, a penetration of 25-50% could be plausible.
- Competitive Landscape: Assume moderate competition with a market share of 20-30%.
- Pricing: For rare disease treatments, annual prices can range from $100,000 to $300,000 per patient.
- Insurance Coverage: Assume a high coverage rate due to the orphan drug status, with 90% of patients covered.
- Dosage and Treatment Duration: Assume chronic treatment with annual patient retention rates around 80-90%.
- Patient Adherence: Assume adherence rates of about 70-80%, given the chronic nature of the condition.
- Gross-to-Net Adjustments: Assume standard discounts and rebates resulting in a net price that is 10-20% lower than the list price.
Using these assumptions, a very rough estimate for peak annual sales could be calculated. However, these figures are purely speculative and should be refined with more specific data as clinical trials progress and more information on the drug's efficacy and market acceptance becomes available.
Obesity
The market opportunity for mitochondrial protonophores in the treatment of obesity is significant due to the rising global incidence of obesity and associated metabolic disorders. Obesity is a major health issue that increases the risk of various diseases, including type 2 diabetes, cardiovascular diseases, and certain cancers.
Traditional weight loss methods, such as lifestyle modification and pharmacotherapy, have limitations in terms of efficacy and sustainability. Therefore, innovative treatments like mitochondrial protonophores that increase energy expenditure could address a considerable unmet need in the obesity treatment landscape.
These agents may offer a new mechanism of action compared to existing therapies, potentially providing additional benefits or serving as an adjunct to current weight management strategies. However, the success of such treatments will depend on their safety profile, efficacy in achieving sustained weight loss, and integration into the broader obesity management protocols.
Revenue build
- Prevalence and Incidence: Assume there are 100 million adults with obesity in the US.
- Diagnosis Rate: If 80% of these are diagnosed, that's 80 million people.
- Market Penetration: Aim for a 1% market penetration in year one, with growth to 3% in seven years.
- Treatment Cost: Assume an annual cost of treatment of $5,000.
- Dosage and Compliance: Estimate a 75% compliance rate.
- Payer Mix: Assume 70% have insurance coverage.
- Discounts and Rebates: Apply a 30% discount/rebate overall.
- Peak Sales: Estimated $5.5B.
These figures could vary widely in reality and should not be used for investment or clinical decisions.
Analyze your company with our AI tools
Get a report like this one for your company or portfolio. Includes excel files with valuation analysis.
Contact us to learn more
TLC-3595 in Type 2 diabetes
Scientific thesis
TLC-3595 is an investigational drug targeting Acetyl-CoA Carboxylase 2 (ACC2), crucial for regulating fatty acid oxidation—a metabolic process where fatty acids break down within mitochondria to produce energy. By inhibiting ACC2, TLC-3595 is designed to increase this oxidation, potentially reducing the ectopic lipid accumulation in non-adipose tissues like the liver and muscles. This ectopic accumulation of lipids is a key driver of insulin resistance, a hallmark of type 2 diabetes. Enhanced fatty acid oxidation can diminish the lipid intermediates that disrupt insulin signaling, thereby improving insulin sensitivity.
Phase 1 trials have revealed TLC-3595's ability to lower LDL and total cholesterol levels significantly without the side effects typical of ACC1 inhibition, such as hypertriglyceridemia and thrombocytopenia. The drug has been well-tolerated, with a pharmacokinetic profile conducive to once-daily dosing.
The relevance of TLC-3595 extends beyond mere symptom management in diabetes; it represents a strategic shift towards rectifying the metabolic dysfunctions underlying insulin resistance. By potentially improving the body's natural response to insulin, TLC-3595 could integrate with existing diabetes treatments, offering a new avenue for managing this chronic disease and its associated risks.
Overview of mechanism of action
Fatty acid oxidation is the metabolic process where fatty acids are broken down to produce energy. This process occurs primarily in the mitochondria, the cell's powerhouse. When the body requires energy, fatty acids are transported into the mitochondria where they undergo beta-oxidation, leading to the production of acetyl-CoA, which then enters the citric acid cycle (Krebs cycle) to generate ATP, the energy currency of the cell.
In healthy individuals, excess fats are stored in adipose tissue. However, when the capacity of adipose tissue to store fat is exceeded or when there is a dysfunction in adipose tissue, fats start to accumulate in these non-adipose tissues (storage of fats in tissues not typically associated with fat storage is called ectopic lipid accumulation), a condition termed lipotoxicity.
This ectopic accumulation of lipids is a key driver of insulin resistance. Fats stored in these non-adipose tissues can interfere with insulin signaling pathways. Specifically, the accumulation of lipid intermediates such as diacylglycerol (DAG) and ceramides can activate a cascade of serine/threonine kinases that phosphorylate and inhibit insulin receptor substrates (IRS). This inhibition impairs the insulin signaling pathway, which is crucial for glucose uptake and metabolism. As a result, the body requires higher levels of insulin to achieve the same glucose uptake, leading to insulin resistance. Over time, the pancreas cannot keep up with the increased demand for insulin, resulting in hyperglycemia and type 2 diabetes.
In essence, by increasing fatty acid oxidation, compounds like TLC-3595 may reduce ectopic lipid levels, thereby potentially restoring normal insulin signaling and improving insulin sensitivity. This therapeutic approach aims to address the metabolic disturbances at the core of insulin resistance, which is central to the development and progression of type 2 diabetes.
The processes involved in this mechanism (fatty acid oxidation, ectopic lipid accumulation, and insulin resistance) are based on well-established biological concepts. These are foundational aspects of metabolism and endocrinology, supported by decades of research. However, there are several key areas where the science continues to evolve and is subject to ongoing research and debate:
- Role of ACC2 in Fatty Acid Oxidation: The role of ACC2 in regulating fatty acid oxidation by controlling the entry of fatty acids into mitochondria is supported by substantial evidence. However, the extent to which inhibition of ACC2 alone can improve insulin sensitivity in humans is less clear, as metabolic regulation is highly complex and redundant.
- Ectopic Lipid Accumulation and Insulin Resistance: The association between ectopic lipid accumulation and insulin resistance is well-supported by numerous studies. The concept that reducing intracellular lipid intermediates can improve insulin sensitivity is widely accepted. However, the detailed mechanisms by which lipids interfere with insulin signaling and the relative contribution of different lipid species to insulin resistance are areas of ongoing research.
- Effects of ACC2 Inhibitors: Preclinical studies have shown promising results with ACC2 inhibitors in animal models. The translation of these findings to humans is a critical step, and the efficacy and safety of these inhibitors in humans are not yet fully established. Phase 1 clinical trial results for compounds like TLC-3595 are encouraging, but these are early-stage trials primarily focused on safety, tolerability, and pharmacokinetics. The results from Phase 2 and Phase 3 trials will be necessary to conclusively demonstrate clinical efficacy and safety.
- Potential Adverse Effects: While the goal is to improve insulin sensitivity without the adverse effects seen with ACC1 inhibition, the full spectrum of effects of selective ACC2 inhibition in the long term is not yet known. It's important to conduct long-term studies to ensure that there are no unintended consequences of chronic ACC2 inhibition.
In summary, while the underlying biology of fatty acid metabolism and insulin resistance is well-established, the development of ACC2 inhibitors like TLC-3595 as a treatment for type 2 diabetes is still a relatively new and evolving field. The overall level of evidence supporting the development of ACC2 inhibitors is strong in terms of the biological rationale and preclinical data, but the definitive clinical evidence from human trials is still forthcoming.
There have been several efforts to study ACC inhibitors in the context of type 2 diabetes and metabolic diseases. The development of inhibitors for both ACC1 and ACC2 has been an area of interest due to their roles in fatty acid metabolism. Here are some relevant findings from these efforts:
- Dual ACC1/ACC2 Inhibitors: Initial efforts focused on the development of dual ACC1 and ACC2 inhibitors, given that both enzymes are involved in lipogenesis and fat oxidation, respectively. However, while these inhibitors showed efficacy in reducing lipogenesis and improving metabolic profiles in preclinical models, they also led to significant side effects, including fatty liver and hypertriglyceridemia, due to the inhibition of ACC1. These side effects have limited the clinical utility of dual ACC inhibitors.
- Selective ACC2 Inhibition: Given the drawbacks of dual ACC inhibition, more recent efforts have shifted towards developing selective ACC2 inhibitors to avoid the ACC1-related side effects. These selective inhibitors are designed to enhance fatty acid oxidation without affecting fatty acid synthesis. In preclinical models, selective ACC2 inhibition has shown promise in reducing ectopic fat deposition and improving insulin sensitivity, making it a promising target for metabolic disorders.
- Clinical Trials: Some clinical trials have been conducted with ACC inhibitors, but the results have been mixed. For example, a study on GS-0976, an ACC inhibitor, showed it reduced liver fat and improved liver biochemistry in NASH patients but also raised triglyceride levels, indicating the challenge of achieving the right balance of ACC1 and ACC2 inhibition.
- Combinatorial Approaches: Considering the complex nature of metabolic diseases, research has also looked into the use of ACC inhibitors in combination with other metabolic drugs. The idea is to enhance the therapeutic effects while minimizing side effects, but this approach requires careful consideration of drug-drug interactions and the cumulative impact on metabolic pathways.
Phase 1 study analysis
The Phase 1 study of TLC-3595 aimed to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of the drug in healthy Japanese male subjects. The study employed a double-blind, placebo-controlled design with single ascending doses (SAD) and multiple ascending doses (MAD) in subjects aged 20–55 years with a BMI of 18.5–30 kg/m^2.
The study included SAD cohorts with doses ranging from 12.5 mg to 400 mg to assess the drug's PK profile and MAD cohorts with doses of 25 mg, 50 mg, and 100 mg for 14 days to assess tolerability and the potential for accumulation. The effect of food on the drug's absorption was also evaluated, which is important as it can inform recommendations for drug administration relative to meals.
Key findings include:
- TLC-3595 showed rapid absorption with a median Tmax (time to peak concentration) of 2 to 5 hours post-dose, and a half-life ranging from 9.5 to 13.3 hours in SAD cohorts and 14 to 17 hours at steady-state in MAD cohorts.
- There was a dose-dependent increase in drug exposure, but with less than dose-proportional increases above 50 mg, suggesting a plateau in absorption or distribution at higher doses.
- TLC-3595 was well tolerated at doses up to 100 mg per day over 14 days, with all treatment-emergent adverse events (TEAEs) being non-serious and mild.
- The drug did not significantly affect plasma triglyceride levels or platelet counts, which suggests selective ACC2 inhibition without significant off-target ACC1 effects.
- There were non-dose dependent reductions in total and LDL cholesterol, indicating a pharmacodynamic effect on lipid metabolism.
High-level analysis:
- The study's design is appropriate for a Phase 1 trial, with a focus on safety and PK/PD profiling.
- The use of a placebo group and a crossover design for the food effect study provides robust controls.
- The sample size is small, which is typical for Phase 1 trials but limits the statistical power to detect rare adverse events or subtle effects.
- The absence of significant changes in triglycerides or platelets suggests good selectivity for ACC2 over ACC1, which is crucial for the intended therapeutic effect without the side effects associated with ACC1 inhibition.
- However, the study's population consisted of healthy volunteers, who may not fully represent the pharmacokinetics or pharmacodynamics in patients with metabolic disorders like type 2 diabetes.
- The results provide a basis for dosing regimens in future trials but do not address the drug's efficacy in treating diabetes or other metabolic diseases.
Overall, the study design is solid for a Phase 1 trial, and the results are promising regarding the safety and potential efficacy of TLC-3595. However, further studies, particularly in patient populations, are necessary to determine the drug's therapeutic value and to confirm its safety profile over longer periods and at varied doses.
The study included a single ascending dose portion and multiple ascending dose portion. Notable aspects of the SAD portion include:
- Demographics
- The inclusion of subjects across a range of BMIs that do not extend into obesity (BMI < 30 kg/m^2) is notable, since TLC-3595 is being developed for metabolic disorders which are often associated with higher BMIs.
- The placebo group includes twice as many subjects as the drug-treated groups, which can provide a stronger comparison for safety and tolerability data.
- Pharmacokinetic Parameters
- A rapid increase in plasma levels is observed post-dosing, with levels peaking at 2 to 5 hours.
- The Area Under the Curve (AUC), which represents the drug's exposure over time, increases with dose but less than proportionally above 50 mg, suggesting saturation of absorption or metabolism at higher doses.
- The maximum concentration (Cmax) also increases with dose and shows a similar pattern to the AUC, with less than proportional increases at higher doses.
- The half-life (t1/2) of the drug does not vary greatly across doses, which suggests consistent elimination kinetics regardless of the dose.
- The data presented for the 50 mg fed cohort indicates that food does not significantly affect the drug's absorption, which is useful for determining dosing instructions.
- Comments
- The study's design in assessing the effect of food is practical and relevant since it can impact the drug's pharmacokinetics and patient compliance.
- The clear dose-dependent pharmacokinetic response up to a certain point is typical for orally administered drugs and is important for determining the optimal dosing range.
- The variability in Tmax and t1/2 at different doses is relatively small, indicating a predictable pharmacokinetic profile, which is favorable for a drug's development.
- The lack of significant increase in drug exposure at the highest doses could imply that the optimal therapeutic dose will be below the maximum tested dose, potentially reducing the risk of adverse effects.
- The demographic data show an appropriate selection of subjects to assess the drug’s PK in a population that could potentially use the medication in the future, although expanding the study to include a broader range of BMIs and both sexes could provide more comprehensive safety data.
Notable aspects of the MAD portion include:
- Demographics
- The study included a relatively small number of participants in each dose group (n=8), which is typical for Phase 1 studies.
- The median age of participants ranged from 23 to 33.5 years across different dosing cohorts, which is a relatively young cohort and does not necessarily reflect the typical age range of type 2 diabetes patients who are generally older.
- Weight and BMI values suggest that participants were within a healthy range and not obese, which is not entirely representative of the general type 2 diabetes population that often has a higher BMI.
- PK Parameter Finding
- The Area Under the Curve (AUC_tau), which represents the overall exposure of the drug over the dosing interval at steady state, shows increases with the dose. The values are 7547 h·ng/mL for 25 mg, 20,430 h·ng/mL for 50 mg, and 26,690 h·ng/mL for 100 mg.
- The AUC accumulation ratios (Day 17 vs. Day 1) are close to 1.5 for all doses, indicating a modest accumulation of the drug upon multiple dosing, which is expected due to the half-life (t_1/2) of the drug being over 12 hours.
- The maximum concentration (C_max) also increases with the dose, and the C_max accumulation ratios are consistent with the AUC ratios, further supporting a modest accumulation.
- The median time to reach maximum concentration (T_max) is between 3.0 to 4.0 hours across all doses, indicating a consistent absorption profile across the dosing range.
- The half-life (t_1/2) is fairly consistent across doses, ranging from 13.8 to 17.4 hours, supporting once-daily dosing.
- Safety Findings
- Treatment-Emergent Adverse Events (TEAEs) were reported in 25% to 38% of subjects across the different dose cohorts, with a similar incidence observed in the placebo group (33%). This suggests that TLC-3595 was well-tolerated with no significant increase in adverse events compared to placebo.
- All reported TEAEs were mild, with no moderate or severe adverse events reported, indicating a favorable safety profile at the tested dose range.
- There were no serious TEAEs and no discontinuations due to TEAEs, further supporting the tolerability of the drug.
- There were related TEAEs reported in the 25 mg and 50 mg TLC-3595 cohorts (25%) as well as the placebo group (25%), suggesting that these may not be directly related to the drug.
- Changes in lipids and platelets
- A decrease in total cholesterol and LDL-C (low-density lipoprotein cholesterol) was observed across all TLC-3595 dose groups (25 mg, 50 mg, and 100 mg) from baseline to the end of treatment. The reductions in total cholesterol and LDL-C are statistically significant at the 100 mg dose when compared with placebo.
- Triglyceride levels increased from baseline in the 25 mg and 50 mg dose groups but decreased in the placebo group. However, these changes were not statistically significant.
- There was a non-significant decrease in platelet counts across all TLC-3595 dose groups and a slight increase in the placebo group.
- Comments
- The absence of serious or severe TEAEs and the lack of treatment discontinuation due to adverse events are encouraging signs in terms of safety for TLC-3595.
- The similar incidence of TEAEs in the drug and placebo groups could suggest a placebo effect or that the observed TEAEs were not related to the drug. However, the small sample size limits the ability to draw firm conclusions.
- The inclusion of only male participants and the lack of diversity in BMI may limit the generalizability of these findings to the broader population of patients with type 2 diabetes, who are often older and have a higher BMI.
- For a Phase 1 study, the primary goal is to evaluate safety and tolerability, and in this context, the study seems to have met its objectives for the doses tested.
- The PK profile demonstrated by the data suggests that TLC-3595 has a relatively predictable and consistent pharmacokinetic profile when given at multiple doses, which is desirable for a chronic medication such as a potential diabetes treatment.
- The modest accumulation of the drug is an important consideration for chronic dosing, as it indicates that the drug will have a stable concentration over time with daily dosing and is unlikely to accumulate to dangerous levels.
- The consistent T_max and t_1/2 across the dosing range provide further evidence that the drug has linear pharmacokinetics at the doses tested, which simplifies dose adjustments and predictions about drug behavior at different doses.
- No significant increase in the AUC or C_max accumulation ratios suggests that there is no disproportionate accumulation of the drug with repeated dosing, which is important for safety.
- The significant decrease in total cholesterol and LDL-C in the TLC-3595 groups, especially at the 100 mg dose, is promising, as high LDL-C is a known risk factor for cardiovascular disease, which is a common comorbidity in type 2 diabetes.
- The increase in triglycerides in the 25 mg and 50 mg dose groups does not seem to follow a dose-response relationship and is not mirrored at the 100 mg dose, suggesting that these changes might not be clinically significant or related to the drug. Moreover, triglyceride levels can be highly variable and influenced by factors like diet, which are not controlled in a Phase 1 study.
- The relative changes in platelets are minimal and within normal variability, suggesting no clinically significant effect of TLC-3595 on platelet count. This is important since ACC1 inhibition can lead to thrombocytopenia, and the lack of significant platelet reduction supports the selectivity of TLC-3595 for ACC2 over ACC1.
- While the changes in lipids and platelets are interesting, they do not directly measure the primary endpoint of a diabetes treatment, which is the control of blood glucose levels. Future studies will need to include measurements of glycemic control, such as fasting blood glucose and HbA1c levels.
- It's also important to note that the study was conducted in healthy volunteers, who typically have normal lipid levels. The drug's effects might differ in patients with type 2 diabetes, who often have dyslipidemia as part of their condition.
Phase 2 study design
OrsoBio is currently enrolling a Phase 2a study of TLC-3595. The Phase 2a study for TLC-3595 is a multicenter, double-blind, randomized trial aiming to assess the safety, tolerability, effectiveness, and pharmacokinetics (PK) of the drug in subjects with insulin resistance. It involves two experimental arms where participants receive one of two doses of TLC-3595 and a comparator arm with a placebo.
Participants are adult men and women (18-70 years) with a BMI of at least 28 kg/m², showing signs of insulin resistance or diagnosed type 2 diabetes. The study excludes individuals with unstable cardiovascular conditions, significant liver disease, recent significant weight loss, and certain other medical or psychological conditions.
The primary outcome measures will be the change in insulin sensitivity as assessed by an oral glucose tolerance test, and the incidence of treatment-emergent adverse events (AEs), evaluated up to day 84 of the study.
The use of insulin sensitivity improvement as measured by oral glucose tolerance test (OGTT) is an appropriate primary endpoint. OGTT is a well-established method for assessing insulin resistance and is directly relevant to the pathophysiology of Type 2 diabetes. Monitoring for treatment-emergent adverse events is a critical safety measure and an appropriate secondary endpoint.
The inclusion criteria are fairly inclusive, capturing a broad age range (18-70 years) and including individuals with a confirmed diagnosis of Type 2 diabetes or insulin resistance (HOMA-IR > 2.84). The requirement for a BMI ≥ 28 kg/m^2 ensures the study addresses those likely to benefit from the treatment, considering obesity is a common feature in Type 2 diabetes.
Some of the exclusion criteria may limit the study's generalizability. For instance, excluding patients with HbA1c ≥ 8% may omit those with poorer glycemic control who could potentially benefit from the drug. Moreover, excluding patients who have had a weight loss of more than 5% in the 90 days prior to screening might exclude individuals actively managing their condition through lifestyle changes. This could potentially limit the applicability of study findings to a typical Type 2 diabetes population.
The relatively stringent inclusion and exclusion criteria may pose challenges for reproducibility in a broader patient population. Real-world patients often have comorbidities and take multiple medications, which may affect the drug's efficacy and safety profile. Furthermore, the exclusion of patients on any treatments for insulin resistance or diabetes in the 90 days prior to screening could limit the study's applicability, as many patients with Type 2 diabetes are on such treatments.
The trial's eligibility criteria are quite comprehensive, potentially limiting the generalizability of the study's findings to a broader patient population with type 2 diabetes. The study's estimated enrollment of 50 may limit its statistical power to detect only the most common or significant effects.
The exclusion of subjects on recent treatments for insulin resistance or diabetes could affect the applicability of the findings to patients who would typically be managing their condition with such medications.
Analyze your company with our AI tools
Get a report like this one for your company or portfolio. Includes excel files with valuation analysis.
Contact us to learn more
TLC-3595 market opportunity
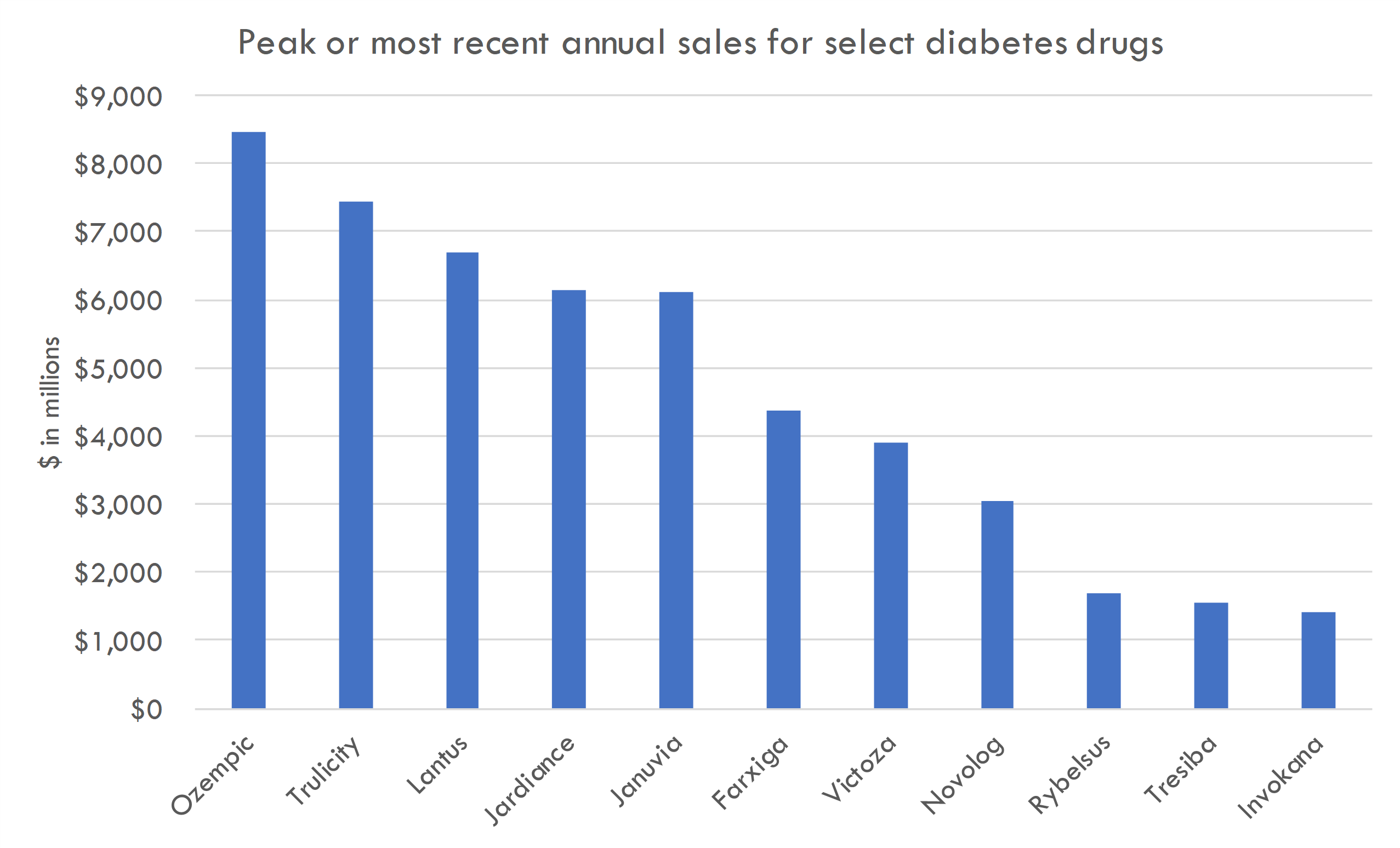
Type 2 diabetes is a chronic metabolic disorder characterized by high levels of sugar (glucose) in the blood due to insulin resistance and relative insulin deficiency. Unlike Type 1 diabetes, where the pancreas produces little or no insulin, in Type 2 diabetes, the body doesn't effectively use the insulin it produces. It is the most common form of diabetes, accounting for around 90-95% of all diabetes cases.
The prevalence of Type 2 diabetes has been increasing globally, a trend that correlates with rising rates of obesity, sedentary lifestyles, and aging populations. It was traditionally seen in adults, but with changing lifestyle patterns, it is now also diagnosed in children and adolescents. The global burden of Type 2 diabetes is substantial and growing, with millions of people affected worldwide and significant associated healthcare costs and economic impact due to its management and treatment of related complications.
Type 2 diabetes drugs have become some of the best-selling drugs in history. For example, Novo Nordisk's Ozempic (semaglutide) is forecasted to reach sales of $12.5 billion in 2023, with projected sales 54% greater than its closest competitor, Trulicity (dulaglutide) by Eli Lilly, which anticipates sales of $8 billion. Other diabetes treatments like Lantus and Januvia were top-selling drugs, with revenues of $6.98 billion and $3.86 billion, respectively, in 2015.
The standard of care for Type 2 diabetes typically begins with lifestyle modifications and metformin. Metformin is used for its glucose-lowering effects and is generally well-tolerated, with a low risk of hypoglycemia and potential benefits for weight management.
For patients who do not achieve glycemic targets with metformin alone, additional therapies include sulfonylureas (which increase insulin secretion from the pancreas), thiazolidinediones (which improve insulin sensitivity), and DPP-4 inhibitors (which enhance the incretin system, which increases insulin release and decreases glucagon levels).
Emerging treatments: GLP-1 agonists and SGLT-2 inhibitors
Emerging treatments include GLP-1 agonists, which have a unique role due to their ability to enhance insulin secretion in a glucose-dependent manner, promote weight loss, and potentially provide cardiovascular benefits. SGLT-2 inhibitors lower glucose by increasing urinary glucose excretion and also have cardiovascular and renal benefits. SGLT-2 inhibitors and GLP-1 agonists have redefined the treatment landscape for Type 2 diabetes due to their benefits in glycemic control, weight reduction, and cardiovascular risk management.
TLC-3595, by targeting ACC2, offers a different mechanism of action focused on enhancing fatty acid oxidation, which could complement the glucose-lowering effects of existing therapies. If clinical trials demonstrate a robust safety profile and efficacy in improving insulin sensitivity and other metabolic outcomes, TLC-3595 could become an attractive option for patients who struggle with insulin resistance, a core defect in Type 2 diabetes.
The unmet need in Type 2 diabetes has evolved with the introduction of these new agents, yet not all patients achieve optimal outcomes with current therapies. There remains a demand for treatments that can provide additional metabolic benefits, potentially with fewer side effects, improved ease of use, and cost-effectiveness. If TLC-3595 can address these points, it could find a place within the multi-faceted treatment approach for Type 2 diabetes.
Limitations of these therapies include:
- GLP-1 Agonists: They may cause gastrointestinal side effects, such as nausea and vomiting. Their injectable form can be less convenient, and not all patients respond to them. Some estimates suggest that up to 20-30% of patients may not have an adequate glycemic response.
- SGLT-2 Inhibitors: Risks include dehydration, genital mycotic infections, and rare but serious complications like diabetic ketoacidosis and Fournier's gangrene. They are also associated with euglycemic ketoacidosis, where ketoacidosis occurs without significantly elevated blood sugar levels.
The actual percentage of responders varies based on individual patient factors, the definition of response used, and the specific agent considered. However, a significant proportion of patients may not achieve target glycemic control, leading to a need for alternative or additional therapies.
Revenue build
We can model a hypothetical revenue build, assuming TLC-3595 is a second-line therapy post-metformin is based on the typical progression of Type 2 diabetes treatment. Metformin is widely recognized as the initial pharmacological treatment for most patients due to its efficacy, safety profile, and cost-effectiveness. When patients fail to achieve glycemic control with metformin alone, additional pharmacotherapy is considered.
Emerging drugs often enter the market as second-line therapies because they need to demonstrate added value over existing first-line treatments. This value can be in the form of better efficacy, reduced side effects, or other benefits like weight loss or cardiovascular protection. Given these factors, it is a strategic starting point for new medications like TLC-3595 to be positioned as an adjunct treatment to metformin.
- Target Population: approximately 30 million people with Type 2 diabetes in the U.S.
- Market Penetration Rate: 0.5% in year one, scaling to 5% in five years.
- Standard of Care Positioning: Second-line therapy post-metformin.
- Competitive Landscape: Assume a modest market share due to established competitors like GLP-1 agonists and SGLT-2 inhibitors.
- Pricing Strategy: $6,000 per patient per year, in line with newer diabetes treatments.
- Treatment Attrition and Adherence: 15% annual attrition, 70% adherence.
- Insurance Coverage: 85% of patients covered by insurance.
- Gross-to-Net Adjustments: 30% discount due to rebates and payer negotiations.
- Revenue Calculation: Number of patients treated x price x adherence rate x coverage rate - discounts.
- Growth Rate: 15% annual growth in patient numbers, tapering as the market matures.
- Peak Sales: Estimated peak sales of $3-4 billion after full market penetration.
This simplified model would serve as a starting point and would require adjustment as real-world data becomes available and as the drug progresses through clinical trials and regulatory review.
TLC-2716 for severe dyslipidemias
Scientific thesis
TLC-2716 is an inhibitor of the Liver X Receptor (LXR), which is central to cholesterol homeostasis and de novo lipogenesis (DNL). LXRs typically promote the expression of genes involved in the synthesis of fatty acids and cholesterol; therefore, inhibiting LXR could theoretically reduce lipid synthesis.
The therapeutic rationale for TLC-2716 as an LXR inverse agonist (inhibitor) is based on its potential to decrease hepatic steatosis and plasma lipids, which are often elevated in conditions like severe hypertriglyceridemia (SHTG) and nonalcoholic steatohepatitis (NASH). The drug has shown promise in preclinical studies, demonstrating reductions in plasma triglycerides and cholesterol. This suggests it may serve as an effective oral therapy for these conditions, which are commonly associated with Type 2 diabetes and are significant risk factors for cardiovascular diseases.
Phase 1 data indicate that TLC-2716 is safe and well-tolerated in healthy volunteers and leads to significant, dose-dependent improvements in atherogenic lipid parameters. These findings, combined with the fact that TLC-2716 did not adversely affect peripheral reverse cholesterol transport due to its liver-targeted action, support further evaluation in patients with metabolic disorders such as SHTG and NASH.
Severe dyslipidemias are a group of disorders characterized by abnormal levels of lipids in the blood, including elevated triglycerides, low-density lipoprotein cholesterol (LDL-C), and/or decreased high-density lipoprotein cholesterol (HDL-C). These conditions can significantly increase the risk of cardiovascular disease.
The causes of severe dyslipidemias are diverse, ranging from genetic factors that lead to disorders like familial hypercholesterolemia, to secondary causes associated with lifestyle factors, diabetes, obesity, and certain medications. Treatment usually involves lifestyle modifications and pharmacotherapy aimed at lowering lipid levels, which may include statins, fibrates, niacin, and newer agents like PCSK9 inhibitors, SGLT-2 inhibitors, and GLP-1 receptor agonists. Severe dyslipidemias often require aggressive treatment due to the high risk of atherosclerotic cardiovascular disease.
Phase 1 study analysis
The Phase 1 study of TLC-2716, an LXR inverse agonist, aimed to evaluate its safety, tolerability, and pharmacokinetics (PK), as well as its pharmacodynamic effects on lipid levels in healthy volunteers. The study used a double-blind, placebo-controlled design with both single ascending dose (SAD) and multiple ascending dose (MAD) cohorts, with attempts to include subjects with elevated triglycerides and LDL-C.
The company posted an abstract with results in November 2023. Key findings include:
- TLC-2716 was rapidly absorbed with a short half-life, supporting liver-targeting pharmacology.
- There were less than dose-proportional increases in plasma exposure.
- TLC-2716 was well-tolerated with mostly mild, gastrointestinal-related TEAEs.
- Significant, dose-dependent improvements in atherogenic lipids were observed, particularly in subjects with higher baseline lipid levels.
The study design was appropriate for early-stage testing, assessing the drug's safety profile and PK characteristics. The focus on hepatic targeting is evident from the rapid absorption and short half-life of the drug. The results suggest that TLC-2716 may lower lipid levels without affecting genes involved in peripheral RCT, indicating potential for treating severe dyslipidemias without the adverse lipid effects seen with systemic LXR agonism. Further evaluation in patients with SHTG and NASH is warranted.
Further detail on the SAD portion of the trial:
- The study enrolled a diverse age range of participants (18-50 years), and the BMI suggests a representative sample of the general population.
- TLC-2716 showed rapid absorption, with Tmax at around 2.5 to 4 hours post-dose across dosages, suggesting quick uptake into the system.
- There was a trend of less than dose-proportional increases in exposure, indicating potential saturation of absorption or first-pass metabolism at higher doses.
- The short half-life of approximately 2 to 4 hours across dosages aligns with the liver-targeted mechanism of TLC-2716, indicating rapid hepatic uptake and clearance.
- The low maximal plasma concentrations correlate with the intended liver specificity, potentially minimizing the risk of off-target effects.
Further detail from the MAD portion of the trial:
- The majority of treatment-emergent adverse events (TEAEs) across all TLC-2716 doses were mild. Common TEAEs included diarrhea, headache, and abdominal pain.
- There was one instance of a Grade 2 TEAE (thrombophlebitis) in the 2 mg dose group deemed unrelated to the study drug.
- There were no serious TEAEs or TEAEs leading to study discontinuation, indicating a favorable safety profile at this stage.
- The drug appears to show dose-dependent improvements in triglycerides (TG), total cholesterol, LDL-C, LDL particles, and ApoB levels, which are important markers of dyslipidemia
- Additionally, the data suggests that TLC-2716 does not suppress the expression of genes involved in peripheral RCT, which is a positive sign as it indicates the drug's selectivity for targeting hepatic lipid metabolism without adversely affecting beneficial cholesterol transport mechanisms.
TLC-2716 market opportunity
Severe dyslipidemias encompass a spectrum of genetic and acquired conditions characterized by abnormal levels of lipids in the blood, notably high total cholesterol, high low-density lipoprotein cholesterol (LDL-C), high triglycerides, and/or low high-density lipoprotein cholesterol (HDL-C). These imbalances can significantly increase the risk of atherosclerotic cardiovascular disease.
The standard of care often includes lifestyle modifications and pharmacotherapy. Statins are the cornerstone for reducing LDL-C levels. Other medications include fibrates, niacin, ezetimibe, and PCSK9 inhibitors. Despite these treatments, some patients, particularly those with genetic lipid disorders, do not achieve optimal lipid control, highlighting a significant unmet clinical need for more effective and targeted therapies. Emerging therapies aim to address this gap by targeting different aspects of lipid metabolism, inflammation, and genetic regulators of lipid homeostasis.
Novel therapies for severe dyslipidemias target various pathways involved in lipid metabolism. These include therapies that:
- Inhibit the synthesis of lipids in the liver
- Increase the clearance of atherogenic lipoproteins from the bloodstream
- Enhance the body's natural mechanisms for removing cholesterol
TLC-2716 fits into this treatment paradigm as a liver-targeted LXR inverse agonist. By inhibiting LXR, it potentially reduces the liver's production of lipids and aids in the clearance of lipoproteins. This mechanism is distinct from that of statins and PCSK9 inhibitors, and it might be particularly beneficial for patients who do not adequately respond to or cannot tolerate current therapies. Given its liver specificity, TLC-2716 may also offer a safety profile that allows for its use in a broader range of patients, including those with hepatic steatosis or non-alcoholic steatohepatitis (NASH), conditions often associated with severe dyslipidemia.
Revenue build
- Target Population: Assume 10 million people with severe dyslipidemia in the US.
- Market Penetration Rate: 1% in year one, increasing to 10% over seven years.
- Competitive Landscape: Moderate competition from statins, PCSK9 inhibitors.
- Pricing Strategy: $4,500 per patient per year.
- Treatment Duration and Compliance: Average treatment duration of 2 years, with a 75% adherence rate.
- Patient Attrition: 10% annual attrition.
- Insurance Coverage: 85% of patients with insurance coverage.
- Gross-to-Net Adjustments: 30% for rebates and discounts.
- Projected Sales: Using the above assumptions to calculate annual sales.
- Peak Sales Estimation: $2 billion at peak after market saturation.
These figures are purely hypothetical and would require validation with actual clinical trial data, market analysis, and more.
ACMSD inhibitor in organ failure
Scientific thesis
ACMSD is an enzyme important for creating NAD+, a molecule vital for the energy production and overall health of cells, especially in the liver and kidneys. In liver and kidney diseases, NAD+ levels are often reduced, leading to impaired mitochondrial function, meaning cells can't produce energy efficiently.
Inhibiting ACMSD can boost NAD+ levels, potentially improving the energy production in cells, and offering protection against tissue damage. This therapeutic approach could potentially address mitochondrial dysfunction that is a characteristic of metabolic syndromes like NASH.
TLC-065, a compound that inhibits ACMSD, has demonstrated in preclinical studies the ability to increase NAD+ levels, enhance fatty acid oxidation, and reduce lipogenesis and oxidative stress in hepatocytes, leading to improved mitochondrial health and reduced lipid content in liver cells. This suggests a strong therapeutic potential for TLC-065 in treating diseases associated with mitochondrial dysfunction, such as liver and kidney disorders.
The science behind ACMSD inhibition to augment NAD+ biosynthesis is still emerging. Although initial studies suggest that increasing NAD+ levels can improve mitochondrial function and protect against liver and kidney injury, this approach is not yet widely established in clinical practice.
The role of ACMSD in NAD+ regulation and its impact on metabolic and inflammatory disorders remain areas of active research. Clinical trials, such as those investigating compounds like TLC-065, are crucial for validating these early findings and establishing the safety and efficacy of ACMSD inhibitors as a therapeutic option. The level of evidence is growing but still largely rests on preclinical studies.
ACMSD inhibitor market opportunity
Liver and kidney diseases encompass a range of conditions that can lead to organ failure, each with distinct patient populations, symptoms, and prognoses.
Liver diseases, such as hepatitis, alcoholic liver disease, and nonalcoholic steatohepatitis (NASH), affect millions worldwide, with cirrhosis being a leading cause of death. These diseases progressively damage the liver leading to fibrosis, cirrhosis, and eventual liver failure. Early stages may be asymptomatic; advanced stages include jaundice, ascites, coagulopathy, and encephalopathy. Prognosis varies from manageable with lifestyle changes and medication to life-threatening, often requiring a liver transplant.
Chronic kidney disease (CKD) affects approximately 15% of adults in the U.S., with a significant portion progressing to end-stage renal disease (ESRD), requiring dialysis or kidney transplantation. CKD is common in patients with diabetes, hypertension, and glomerulonephritis. Symptoms include edema, fatigue, and uremia. Prognosis can worsen without intervention, with dialysis or transplant as the only options in ESRD.
Both conditions represent significant burdens on healthcare systems and have high unmet medical needs, especially in delaying progression to organ failure and improving quality of life.
The standard of care for liver and kidney diseases includes lifestyle changes, medications, and in severe cases, organ transplantation:
In liver disease, the standard of care includes medications to manage symptoms, antiviral drugs for hepatitis, and lifestyle changes such as abstaining from alcohol and maintaining a healthy weight. There is a need for effective treatments for early-stage liver disease, therapies to reverse fibrosis, and alternatives to transplantation.
Treatments for kidney disease include blood pressure control, blood sugar management in diabetics, specific diets, and medications to manage complications; as well as dialysis or transplantation in ESRD. Unmet medical needs include treatments that slow CKD progression, improve dialysis outcomes, and reduce the need for transplantation.
An ACMSD inhibitor that augments NAD+ biosynthesis and improves mitochondrial function could represent a novel therapeutic approach in the treatment of liver and kidney dysfunctions. By potentially enhancing cellular energy metabolism and reducing oxidative stress, it may address the underlying mitochondrial dysfunctions associated with these diseases. This could complement existing treatments, aiming to slow disease progression, improve organ function, and reduce the need for transplants. However, such treatments would need to demonstrate efficacy and safety in clinical trials before becoming part of the standard care regimen.
Analyze your company with our AI tools
Get a report like this one for your company or portfolio. Includes excel files with valuation analysis.
Contact us to learn more